Examining the management of muscle-invasive bladder cancer by medical oncologists in the United States
- PMID: 24840869
- PMCID: PMC6771274
- DOI: 10.1016/j.urolonc.2013.12.012
Examining the management of muscle-invasive bladder cancer by medical oncologists in the United States
Abstract
Background: Neoadjuvant chemotherapy (NACT) for the treatment of muscle-invasive bladder cancer (MIBC) remains underutilized in the United States despite evidence supporting its use.
Objectives: To examine the perioperative chemotherapy management of patients with MIBC by medical oncologists (MedOncs) to move toward standardization of practice
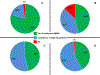
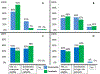
Participants and methods: A 26-question survey was emailed to 92 MedOncs belonging to the Bladder Cancer Advocacy Network or the American Society of Clinical Oncology for completion from May to October 2011 RESULTS: A total of 83 MedOncs completed the survey: 52% were based in academic centers. Most referrals were from urologists (79%). NACT for treatment of MIBC and high-grade upper-tract urothelial carcinoma is offered by 80% and 46% of respondents, respectively. Adjuvant chemotherapy for treatment of MIBC and upper-tract urothelial carcinoma is offered by 46% and 42% of respondents, respectively. NACT was not offered by 49%, 29%, and 35% of respondents if Eastern Cooperative Oncology Group performance status was 3 or greater, if patients had T2 lesions without lymphovascular invasion, and if the glomerular filtration rate was<50ml/min, respectively. Chemotherapy regimens included gemcitabine/cisplatin (90%), methotrexate/vinblastine/adriamycin/cisplatin (30%), dose-dense methotrexate, vinblastine, adriamycin, and cisplatin (20%), and gemcitabine/carboplatin (37%).
Conclusions: Most MedOncs (79%) in this survey offer perioperative chemotherapy to all patients with MIBC. This increased use of NACT is higher than previously reported, suggesting an increase in the adoption of recommendations that follow best evidence.
Keywords: Adjuvant chemotherapy; Medical oncologist; Muscle-invasive bladder cancer; Neoadjuvant chemotherapy; Survey.
Published by Elsevier Inc.
Figures


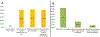
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10–29. - PubMed
-
- Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 2005;66:4–34. - PubMed
-
- Skinner DG, Lieskovsky G. Contemporary cystectomy with pelvic node dissection compared to preoperative radiation therapy plus cystectomy in management of invasive bladder cancer. J Urol 1984;131:1069–72. - PubMed
-
- Herr HW, Dotan Z, Donat SM, Bajorin DF. Defining optimal therapy for muscle invasive bladder cancer. J Urol 2007;177:437–43. - PubMed
-
- International Collaboration of T, Medical Research Council Advanced Bladder Cancer Working P, European Organisation for R, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol 2011; 29:2171–7. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

