Utilization of acidic α-amino acids as acyl donors: an effective stereo-controllable synthesis of aryl-keto α-amino acids and their derivatives
- PMID: 24840903
- PMCID: PMC6271428
- DOI: 10.3390/molecules19056349
Utilization of acidic α-amino acids as acyl donors: an effective stereo-controllable synthesis of aryl-keto α-amino acids and their derivatives
Abstract
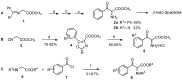
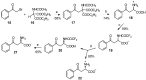
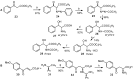
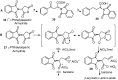
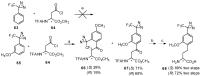
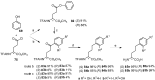
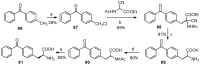
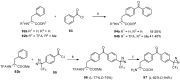
Aryl-keto-containing α-amino acids are of great importance in organic chemistry and biochemistry. They are valuable intermediates for the construction of hydroxyl α-amino acids, nonproteinogenic α-amino acids, as well as other biofunctional components. Friedel-Crafts acylation is an effective method to prepare aryl-keto derivatives. In this review, we summarize the preparation of aryl-keto containing α-amino acids by Friedel-Crafts acylation using acidic α-amino acids as acyl-donors and Lewis acids or Brönsted acids as catalysts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





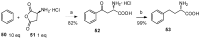



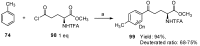
Similar articles
-
Synthesis of Chiral TFA-Protected α-Amino Aryl-Ketone Derivatives with Friedel-Crafts Acylation of α-Amino Acid N-Hydroxysuccinimide Ester.Molecules. 2017 Oct 17;22(10):1748. doi: 10.3390/molecules22101748. Molecules. 2017. PMID: 29039791 Free PMC article.
-
Tunable Aryl Imidazolium Recyclable Ionic Liquid with Dual Brønsted-Lewis Acid as Green Catalyst for Friedel-Crafts Acylation and Thioesterification.Molecules. 2020 Jan 15;25(2):352. doi: 10.3390/molecules25020352. Molecules. 2020. PMID: 31952217 Free PMC article.
-
Friedel-Crafts Acylation of Aminocarboxylic Acids in Strong Brønsted Acid Promoted by Lewis Base P4O10.J Org Chem. 2022 Nov 18;87(22):15224-15249. doi: 10.1021/acs.joc.2c01761. Epub 2022 Nov 1. J Org Chem. 2022. PMID: 36318089
-
Friedel-Crafts reactions for biomolecular chemistry.Org Biomol Chem. 2024 May 8;22(18):3544-3558. doi: 10.1039/d4ob00406j. Org Biomol Chem. 2024. PMID: 38624091 Review.
-
Biocatalytic Friedel-Crafts Reactions.ChemCatChem. 2022 Sep 20;14(18):e202200636. doi: 10.1002/cctc.202200636. Epub 2022 Aug 24. ChemCatChem. 2022. PMID: 36606067 Free PMC article. Review.
Cited by
-
Synthesis, In Silico and In Vitro Evaluation of Antimicrobial and Toxicity Features of New 4-[(4-Chlorophenyl)sulfonyl]benzoic Acid Derivatives.Molecules. 2021 Aug 23;26(16):5107. doi: 10.3390/molecules26165107. Molecules. 2021. PMID: 34443693 Free PMC article.
-
Synthesis of Chiral TFA-Protected α-Amino Aryl-Ketone Derivatives with Friedel-Crafts Acylation of α-Amino Acid N-Hydroxysuccinimide Ester.Molecules. 2017 Oct 17;22(10):1748. doi: 10.3390/molecules22101748. Molecules. 2017. PMID: 29039791 Free PMC article.
References
-
- Hartung W.H., Dittrich T.T., Chang Y.T. β-Arylserine ethyl esters. J. Am. Chem. Soc. 1953;75:238–239. doi: 10.1021/ja01097a513. - DOI
-
- Hernández D., Vilar G., Riego E., Cañedo L.M., Cuevas C., Albericio F., Álvarez M. Synthesis of IB-01211, a cyclic peptide containing 2,4-concatenated thia- and oxazoles, via Hantzsch macrocyclization. Org. Lett. 2007;9:809–811. - PubMed
-
- Vanderhaeghe H., Parmentier G. The structure of factor S of staphylomycin. J. Am. Chem. Soc. 1960;82:4414–4422. doi: 10.1021/ja01501a070. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

