Mitochondrial NLRP3 protein induces reactive oxygen species to promote Smad protein signaling and fibrosis independent from the inflammasome
- PMID: 24841199
- PMCID: PMC4094069
- DOI: 10.1074/jbc.M114.550624
Mitochondrial NLRP3 protein induces reactive oxygen species to promote Smad protein signaling and fibrosis independent from the inflammasome
Abstract
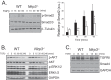
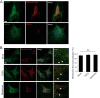
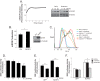
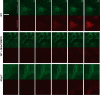
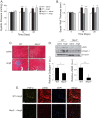
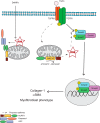
Nucleotide-binding domain and leucine-rich repeat containing PYD-3 (NLRP3) is a pattern recognition receptor that is implicated in the pathogenesis of inflammation and chronic diseases. Although much is known regarding the NLRP3 inflammasome that regulates proinflammatory cytokine production in innate immune cells, the role of NLRP3 in non-professional immune cells is unclear. Here we report that NLRP3 is expressed in cardiac fibroblasts and increased during TGFβ stimulation. NLRP3-deficient cardiac fibroblasts displayed impaired differentiation and R-Smad activation in response to TGFβ. Only the central nucleotide binding domain of NLRP3 was required to augment R-Smad signaling because the N-terminal Pyrin or C-terminal leucine-rich repeat domains were dispensable. Interestingly, NLRP3 regulation of myofibroblast differentiation proceeded independently from the inflammasome, IL-1β/IL-18, or caspase 1. Instead, mitochondrially localized NLRP3 potentiated reactive oxygen species to augment R-Smad activation. In vivo, NLRP3-deficient mice were protected against angiotensin II-induced cardiac fibrosis with preserved cardiac architecture and reduced collagen 1. Together, these results support a distinct role for NLRP3 in non-professional immune cells independent from the inflammasome to regulate differential aspects of wound healing and chronic disease.
Keywords: Fibrosis; Heart Failure; Inflammation; Myofibroblast; Transforming Growth Factor β (TGFβ).
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

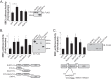


References
-
- van den Borne S. W., Diez J., Blankesteijn W. M., Verjans J., Hofstra L. (2010) Myocardial remodeling after infarction: the role of myofibroblasts. Nat. Rev. Cardiol. 7, 30–37 - PubMed
-
- Petrov V. V, Fagard R. H., Lijnen P. J. (2002) Stimulation of collagen production by transforming growth factor-B1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension 39, 258–263 - PubMed
-
- Di Guglielmo G. M., Le Roy C., Goodfellow A. F., Wrana J. L. (2003) Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat. Cell Biol. 5, 410–421 - PubMed
-
- Xu L., Chen Y. G., Massagué J. (2000) The nuclear import function of Smad2 is masked by SARA and unmasked by TGFβ-dependent phosphorylation. Nat. Cell Biol. 2, 559–562 - PubMed
-
- Wrana J. L., Attisano L., Wieser R., Ventura F., Massagué J. (1994) Mechanism of activation of the TGFβ receptor. Nature 370, 341–347 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

