Antitrypanosomal activity of fexinidazole metabolites, potential new drug candidates for Chagas disease
- PMID: 24841257
- PMCID: PMC4136024
- DOI: 10.1128/AAC.02754-13
Antitrypanosomal activity of fexinidazole metabolites, potential new drug candidates for Chagas disease
Abstract
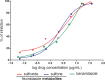
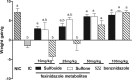
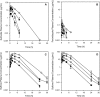
This study was designed to verify the in vivo efficacy of sulfoxide and sulfone fexinidazole metabolites following oral administration in a murine model of Chagas disease. Female Swiss mice infected with the Y strain of Trypanosoma cruzi were treated orally once per day with each metabolite at doses of 10 to 100 mg/kg of body weight for a period of 20 days. Parasitemia was monitored throughout, and cures were detected by parasitological and PCR assays. The results were compared with those achieved with benznidazole treatment at the same doses. Fexinidazole metabolites were effective in reducing the numbers of circulating parasites and protecting mice against death, compared with untreated mice, but without providing cures at daily doses of 10 and 25 mg/kg. Both metabolites were effective in curing mice at 50 mg/kg/day (30% to 40%) and 100 mg/kg/day (100%). In the benznidazole-treated group, parasitological cure was detected only in animals treated with the higher dose of 100 mg/kg/day (80%). Single-dose pharmacokinetic parameters for each metabolite were obtained from a parallel group of uninfected mice and were used to estimate the profiles following repeated doses. Pharmacokinetic data suggested that biological efficacy most likely resides with the sulfone metabolite (or subsequent reactive metabolites formed following reduction of the nitro group) following administration of either the sulfoxide or the sulfone and that prolonged plasma exposure over the 24-h dosing window is required to achieve high cure rates. Fexinidazole metabolites were effective in treating T. cruzi in a mouse model of acute infection, with cure rates superior to those achieved with either fexinidazole itself or benznidazole.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Raether W, Seidenath H. 1983. The activity of fexinidazole (HOE 239) against experimental infections with Trypanosoma cruzi, Trichomonas and Entamoeba histolytica. Ann. Trop. Med. Parasitol. 77:13–26 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

