Interaction between mutations and regulation of gene expression during development of de novo antibiotic resistance
- PMID: 24841263
- PMCID: PMC4135992
- DOI: 10.1128/AAC.02892-14
Interaction between mutations and regulation of gene expression during development of de novo antibiotic resistance
Abstract
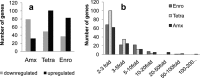
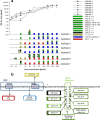
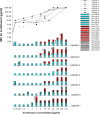
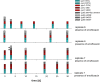
Bacteria can become resistant not only by horizontal gene transfer or other forms of exchange of genetic information but also by de novo by adaptation at the gene expression level and through DNA mutations. The interrelationship between changes in gene expression and DNA mutations during acquisition of resistance is not well documented. In addition, it is not known whether the DNA mutations leading to resistance always occur in the same order and whether the final result is always identical. The expression of >4,000 genes in Escherichia coli was compared upon adaptation to amoxicillin, tetracycline, and enrofloxacin. During adaptation, known resistance genes were sequenced for mutations that cause resistance. The order of mutations varied within two sets of strains adapted in parallel to amoxicillin and enrofloxacin, respectively, whereas the buildup of resistance was very similar. No specific mutations were related to the rather modest increase in tetracycline resistance. Ribosome-sensed induction and efflux pump activation initially protected the cell through induction of expression and allowed it to survive low levels of antibiotics. Subsequently, mutations were promoted by the stress-induced SOS response that stimulated modulation of genetic instability, and these mutations resulted in resistance to even higher antibiotic concentrations. The initial adaptation at the expression level enabled a subsequent trial and error search for the optimal mutations. The quantitative adjustment of cellular processes at different levels accelerated the acquisition of antibiotic resistance.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

