Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection
- PMID: 24841273
- PMCID: PMC4136000
- DOI: 10.1128/AAC.03036-14
Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection
Abstract
Outbreaks of emerging infections present health professionals with the unique challenge of trying to select appropriate pharmacologic treatments in the clinic with little time available for drug testing and development. Typically, clinicians are left with general supportive care and often untested convalescent-phase plasma as available treatment options. Repurposing of approved pharmaceutical drugs for new indications presents an attractive alternative to clinicians, researchers, public health agencies, drug developers, and funding agencies. Given the development times and manufacturing requirements for new products, repurposing of existing drugs is likely the only solution for outbreaks due to emerging viruses. In the studies described here, a library of 290 compounds was screened for antiviral activity against Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus (SARS-CoV). Selection of compounds for inclusion in the library was dependent on current or previous FDA approval or advanced clinical development. Some drugs that had a well-defined cellular pathway as target were included. In total, 27 compounds with activity against both MERS-CoV and SARS-CoV were identified. The compounds belong to 13 different classes of pharmaceuticals, including inhibitors of estrogen receptors used for cancer treatment and inhibitors of dopamine receptor used as antipsychotics. The drugs identified in these screens provide new targets for in vivo studies as well as incorporation into ongoing clinical studies.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
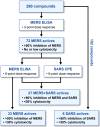
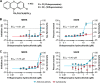
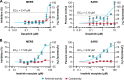
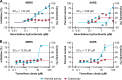
References
-
- WHO. 2003. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. http://www.who.int/csr/sars/country/table2004_04_21/en/index.html
-
- The WHO MERS-Cov Research Group. 12 November 2013. State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. PLoS Curr. 5:pii:ecurrents.outbreaks.0bf719e352e7478f7478ad7485fa30127ddb30128. 10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8 - DOI - PMC - PubMed
-
- Raj VS, Mou H, Smits SL, Dekkers DH, Muller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, Thiel V, Drosten C, Rottier PJ, Osterhaus AD, Bosch BJ, Haagmans BL. 2013. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 495:251–254. 10.1038/nature12005 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

