T cells redirected to interleukin-13Rα2 with interleukin-13 mutein--chimeric antigen receptors have anti-glioma activity but also recognize interleukin-13Rα1
- PMID: 24841514
- PMCID: PMC4087074
- DOI: 10.1016/j.jcyt.2014.02.012
T cells redirected to interleukin-13Rα2 with interleukin-13 mutein--chimeric antigen receptors have anti-glioma activity but also recognize interleukin-13Rα1
Abstract
Background aims: Outcomes for patients with glioblastoma remain poor despite aggressive multimodal therapy. Immunotherapy with genetically modified T cells expressing chimeric antigen receptors (CARs) targeting interleukin (IL) 13Rα2, human epidermal growth factor receptor 2, epidermal growth factor variant III or erythropoietin-producing hepatocellular carcinoma A2 has shown promise for the treatment of glioma in preclinical models. On the basis of IL13Rα2 immunotoxins that contain IL13 molecules with one or two amino acid substitutions (IL13 muteins) to confer specificity to IL13Rα2, investigators have constructed CARS with IL13 muteins as antigen-binding domains. Whereas the specificity of IL13 muteins in the context of immunotoxins is well characterized, limited information is available for CAR T cells.
Methods: We constructed four second-generation CARs with IL13 muteins with one or two amino acid substitutions, and evaluated the effector function of IL13-mutein CAR T cells in vitro and in vivo.
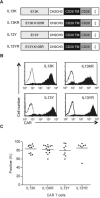
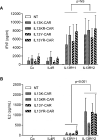
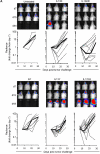
Results: T cells expressing all four CARs recognized IL13Rα1 or IL13Rα2 recombinant protein in contrast to control protein (IL4R) as judged by interferon-γ production. IL13 protein produced significantly more IL2, indicating that IL13 mutein-CAR T cells have a higher affinity to IL13Rα2 than to IL13Rα1. In cytotoxicity assays, CAR T cells killed IL13Rα1- and/or IL13Rα2-positive cells in contrast to IL13Rα1- and IL13Rα2-negative controls. Although we observed no significant differences between IL13 mutein-CAR T cells in vitro, only T cells expressing IL13 mutein-CARs with an E13K amino acid substitution had anti-tumor activity in vivo that resulted in a survival advantage of treated animals.
Conclusions: Our study highlights that the specificity/avidity of ligands is context-dependent and that evaluating CAR T cells in preclinical animal model is critical to assess their potential benefit.
Keywords: CAR; GBM; IL13Rα1; IL13Rα2; T cells; cancer; immunotherapy.
Copyright © 2014 International Society for Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Figures




Comment in
-
A closer look at chimeric antigen receptor specificity.Cytotherapy. 2014 Oct;16(10):1323-4. doi: 10.1016/j.jcyt.2014.08.002. Epub 2014 Sep 16. Cytotherapy. 2014. PMID: 26340442 No abstract available.
References
-
- Finocchiaro G, Pellegatta S. Immunotherapy for glioma: getting closer to the clinical arena? Curr Opin Neurol. 2011;24:641–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

