Tiotropium in patients with moderate COPD naive to maintenance therapy: a randomised placebo-controlled trial
- PMID: 24841833
- PMCID: PMC4373257
- DOI: 10.1038/npjpcrm.2014.3
Tiotropium in patients with moderate COPD naive to maintenance therapy: a randomised placebo-controlled trial
Abstract
Background: The benefits of pharmacotherapy with tiotropium HandiHaler 18 μg for patients with chronic obstructive pulmonary disease (COPD) have been previously demonstrated. However, few data exist regarding the treatment of moderate disease (Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage II).
Aims: To determine whether tiotropium improves lung function/patient-reported outcomes in patients with GOLD stage II COPD naive to maintenance therapy.
Methods: A randomised 24-week double-blind placebo-controlled trial of tiotropium 18 μg once daily (via HandiHaler) was performed in maintenance therapy-naive patients with forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio <0.7 and post-bronchodilator FEV1 ≥50 and <80%.
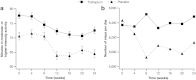
Results: A total of 457 patients were randomised (238 tiotropium, 219 placebo; mean age 62 years; FEV1 1.93 l (66% predicted)). Tiotropium was superior to placebo in mean change from baseline in post-dose FEV1 area under the curve from 0 to 3 h (AUC0-3h) at week 24 (primary endpoint): 0.19 vs. -0.03 l (least-squares mean difference 0.23 l, P<0.001). FVC AUC0-3h, trough and peak FEV1 and FVC were significantly improved with tiotropium versus placebo (P<0.001). Compared with placebo, tiotropium provided numerical improvements in physical activity (P=NS). Physician's Global Assessment (health status) improved (P=0.045) with less impairment on the Work Productivity and Activity Impairment questionnaire (P=0.043) at week 24. The incidence of exacerbations, cough, bronchitis and dyspnoea was lower with tiotropium than placebo.
Conclusions: Tiotropium improved lung function and patient-reported outcomes in maintenance therapy-naive patients with GOLD stage II COPD, suggesting benefits in initiating maintenance therapy early.
Trial registration: ClinicalTrials.gov NCT00523991.
Figures



Comment in
-
The difficulties of measuring and improving physical activity in COPD.NPJ Prim Care Respir Med. 2014 May 20;24:14014. doi: 10.1038/npjpcrm.2014.14. NPJ Prim Care Respir Med. 2014. PMID: 24841568 Free PMC article. No abstract available.
References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the Diagnosis, Management and Prevention of COPD . http://www.goldcopd.org/ 2013
-
- Adams SG, Anzueto A, Briggs DD, Jr, Menjoge SS, Kesten S. Tiotropium in COPD patients not previously receiving maintenance respiratory medications. Respir Med. 2006;100:1495–1503. - PubMed
-
- Kornmann O, Beeh KM, Beier J, Geis UP, Ksoll M, Buhl R. Newly diagnosed chronic obstructive pulmonary disease. Clinical features and distribution of the novel stages of the Global Initiative for Obstructive Lung Disease. Respiration. 2003;70:67–75. - PubMed
-
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554. - PubMed
-
- Niewoehner DE, Rice K, Cote C, Paulson D, Cooper JA, Jr, Korducki L. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005;143:317–326. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

