Geometric consequences of electron delocalization for adenine tautomers in aqueous solution
- PMID: 24842324
- PMCID: PMC4072068
- DOI: 10.1007/s00894-014-2234-4
Geometric consequences of electron delocalization for adenine tautomers in aqueous solution
Abstract
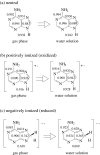
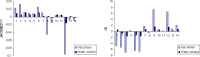
Geometric consequences of electron delocalization were studied for all possible adenine tautomers in aqueous solution by means of ab initio methods {PCM(water)//DFT(B3LYP)/6-311+G(d,p)} and compared to those in the gas phase {DFT(B3LYP)/6-311+G(d,p)}. To measure the consequences of any type of resonance conjugation (π-π, n-π, and σ-π), the geometry-based harmonic oscillator model of electron delocalization (HOMED) index, recently extended to the isolated (DFT) and hydrated (PCM//DFT) molecules, was applied to the molecular fragments (imidazole, pyrimidine, 4-aminopyrimidine, and purine) and also to the whole tautomeric system. For individual tautomers, the resonance conjugations and consequently the bond lengths strongly depend on the position of the labile protons. The HOMED indices are larger for tautomers (or their fragments) possessing the labile proton(s) at the N rather than C atom. Solvent interactions with adenine tautomers slightly increase the resonance conjugations. Consequently, they slightly shorten the single bonds and lengthen the double bonds. When going from the gas phase to water solution, the HOMED indices increase (by less than 0.15 units). There is a good relation between the HOMED indices estimated in water solution and those in the gas phase for the neutral and ionized forms of adenine. Subtle effects, being a consequence of intramolecular interactions between the neighboring groups, are so strongly reduced by solvent that the relation between the HOMED indices and the relative energies for the neutral adenine tautomers seems to be better in water solution than in the gas phase.
Figures




Similar articles
-
Variations of the tautomeric preferences and π-electron delocalization for the neutral and redox forms of purine when proceeding from the gas phase (DFT) to water (PCM).J Mol Model. 2013 Sep;19(9):3947-60. doi: 10.1007/s00894-013-1926-5. Epub 2013 Jul 7. J Mol Model. 2013. PMID: 23832652 Free PMC article.
-
Purine tautomeric preferences and bond-length alternation in relation with protonation-deprotonation and alkali metal cationization.J Mol Model. 2020 Apr 4;26(5):93. doi: 10.1007/s00894-020-4343-6. J Mol Model. 2020. PMID: 32248379 Free PMC article.
-
Quantum-chemical studies on the favored and rare tautomers of neutral and redox adenine.J Phys Chem A. 2013 Feb 21;117(7):1548-59. doi: 10.1021/jp3081029. Epub 2013 Feb 5. J Phys Chem A. 2013. PMID: 23347296
-
On Prototropy and Bond Length Alternation in Neutral and Ionized Pyrimidine Bases and Their Model Azines in Vacuo.Molecules. 2023 Oct 26;28(21):7282. doi: 10.3390/molecules28217282. Molecules. 2023. PMID: 37959699 Free PMC article. Review.
-
Computational study of the dimerization of glyphosate: mechanism and effect of solvent.RSC Adv. 2024 Jul 23;14(32):23184-23203. doi: 10.1039/d4ra04300f. eCollection 2024 Jul 19. RSC Adv. 2024. PMID: 39045405 Free PMC article. Review.
Cited by
-
Influence of the Solvent on the Stability of Aminopurine Tautomers and Properties of the Amino Group.Molecules. 2023 Mar 27;28(7):2993. doi: 10.3390/molecules28072993. Molecules. 2023. PMID: 37049758 Free PMC article.
-
Purinergic system in cancer stem cells.Purinergic Signal. 2025 Feb;21(1):23-38. doi: 10.1007/s11302-023-09976-5. Epub 2023 Nov 15. Purinergic Signal. 2025. PMID: 37966629 Free PMC article. Review.
-
Effect of the Solvent and Substituent on Tautomeric Preferences of Amine-Adenine Tautomers.ACS Omega. 2021 Jul 12;6(29):18890-18903. doi: 10.1021/acsomega.1c02118. eCollection 2021 Jul 27. ACS Omega. 2021. PMID: 34337229 Free PMC article.
-
Impact of the Substituents on the Electronic Structure of the Four Most Stable Tautomers of Purine and Their Adenine Analogues.ACS Omega. 2020 May 12;5(20):11570-11577. doi: 10.1021/acsomega.0c00820. eCollection 2020 May 26. ACS Omega. 2020. PMID: 32478247 Free PMC article.
References
-
- Elguero J, Marzin C, Katritzky AR, Linda P (1976) The tautomerism of heterocycles. Academic, New York
-
- Pozharskii AF, Soldatenkov AT, Katritzky AR. Heterocycles in life and society. New York: Wiley; 1997.
-
- Kwiatkowski JS, Person WB. In: Theoretical biochemistry and molecular biology. Beveridge DL, Lavery R, editors. Academic: New York; 1990.
-
- Pauling L. The Nature of the Chemical Bonds. 3. New York: Cornell University Press; 1960.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

