Routine echocardiography screening for asymptomatic left ventricular dysfunction in childhood cancer survivors: a model-based estimation of the clinical and economic effects
- PMID: 24842413
- PMCID: PMC4089868
- DOI: 10.7326/M13-2266
Routine echocardiography screening for asymptomatic left ventricular dysfunction in childhood cancer survivors: a model-based estimation of the clinical and economic effects
Abstract
Background: Childhood cancer survivors treated with cardiotoxic therapies are recommended to have routine cardiac assessment every 1 to 5 years, but the long-term benefits are uncertain.
Objective: To estimate the cost-effectiveness of routine cardiac assessment to detect asymptomatic left ventricular dysfunction and of angiotensin-converting enzyme inhibitor and β-blocker treatment to reduce congestive heart failure (CHF) incidence in childhood cancer survivors.
Design: Simulation model.
Data sources: Literature, including data from the Childhood Cancer Survivor Study.
Target population: Childhood cancer survivors.
Time horizon: Lifetime.
Perspective: Societal.
Intervention: Interval-based echocardiography assessment every 1, 2, 5, or 10 years, with subsequent angiotensin-converting enzyme inhibitor or β-blocker treatment for patients with positive test results.
Outcome measures: Lifetime risk for systolic CHF, lifetime costs, quality-adjusted life expectancy, and incremental cost-effectiveness ratios (ICERs).
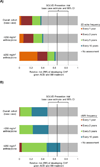
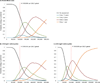
Results of base-case analysis: The lifetime risk for systolic CHF among 5-year childhood cancer survivors aged 15 years was 18.8% without routine cardiac assessment (average age at onset, 58.8 years). Routine echocardiography reduced lifetime risk for CHF by 2.3% (with assessment every 10 years) to 8.7% (annual assessment). The ICER for assessment every 10 years was $111 600 per quality-adjusted life-year (QALY) compared with no assessment. Assessment every 5 years had an ICER of $117 900 per QALY, and ICERs for more frequent assessment exceeded $165 000 per QALY.
Results of sensitivity analysis: Results were sensitive to treatment effectiveness, absolute excess risk for CHF, and asymptomatic left ventricular dysfunction asymptomatic period. The probability that assessment every 10 or 5 years was preferred at a $100 000-per-QALY threshold was 0.33 for the overall cohort.
Limitation: Treatment effectiveness was based on adult data.
Conclusion: Current recommendations for cardiac assessment may reduce CHF incidence, but less frequent assessment may be preferable.
Figures



Comment in
-
Cost-effectiveness of screening for asymptomatic left ventricular dysfunction in childhood cancer survivors.Ann Intern Med. 2014 May 20;160(10):731-2. doi: 10.7326/M14-0823. Ann Intern Med. 2014. PMID: 24842420 No abstract available.
Similar articles
-
Cost-effectiveness of the children's oncology group long-term follow-up screening guidelines for childhood cancer survivors at risk for treatment-related heart failure.Ann Intern Med. 2014 May 20;160(10):672-83. doi: 10.7326/M13-2498. Ann Intern Med. 2014. PMID: 24842414 Free PMC article.
-
Cost-Effectiveness of the International Late Effects of Childhood Cancer Guideline Harmonization Group Screening Guidelines to Prevent Heart Failure in Survivors of Childhood Cancer.J Clin Oncol. 2020 Nov 20;38(33):3851-3862. doi: 10.1200/JCO.20.00418. Epub 2020 Aug 14. J Clin Oncol. 2020. PMID: 32795226 Free PMC article.
-
Cost-effectiveness of screening for asymptomatic left ventricular dysfunction in childhood cancer survivors.Ann Intern Med. 2014 May 20;160(10):731-2. doi: 10.7326/M14-0823. Ann Intern Med. 2014. PMID: 24842420 No abstract available.
-
Angiotensin converting enzyme (ACE) inhibitors and heart failure. The consequences of underprescribing.Pharmacoeconomics. 1999 Jun;15(6):535-50. doi: 10.2165/00019053-199915060-00002. Pharmacoeconomics. 1999. PMID: 10538327 Review.
-
Management of asymptomatic left ventricular dysfunction.Cleve Clin J Med. 2001 Mar;68(3):249-55. doi: 10.3949/ccjm.68.3.249. Cleve Clin J Med. 2001. PMID: 11263853 Review.
Cited by
-
Improved Cardiomyopathy Risk Prediction Using Global Longitudinal Strain and N-Terminal-Pro-B-Type Natriuretic Peptide in Survivors of Childhood Cancer Exposed to Cardiotoxic Therapy.J Clin Oncol. 2024 Apr 10;42(11):1265-1277. doi: 10.1200/JCO.23.01796. Epub 2024 Jan 11. J Clin Oncol. 2024. PMID: 38207238 Free PMC article.
-
Cardiovascular prevention in the cancer survivor.Curr Atheroscler Rep. 2015 Mar;17(3):484. doi: 10.1007/s11883-014-0484-3. Curr Atheroscler Rep. 2015. PMID: 25637040 Review.
-
Survivorship, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology.J Natl Compr Canc Netw. 2018 Oct;16(10):1216-1247. doi: 10.6004/jnccn.2018.0078. J Natl Compr Canc Netw. 2018. PMID: 30323092 Free PMC article.
-
Bending the Cost Curve in Childhood Cancer.Curr Hematol Malig Rep. 2016 Aug;11(4):295-302. doi: 10.1007/s11899-016-0332-3. Curr Hematol Malig Rep. 2016. PMID: 27193602 Review.
-
Paediatric cardio-oncology: epidemiology, screening, prevention, and treatment.Cardiovasc Res. 2019 Apr 15;115(5):922-934. doi: 10.1093/cvr/cvz031. Cardiovasc Res. 2019. PMID: 30768157 Free PMC article. Review.
References
-
- Mertens AC, Yasui Y, Neglia JP, Potter JD, Nesbit ME, Jr, Ruccione K, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2001;19(13):3163–3172. - PubMed
-
- Moller TR, Garwicz S, Barlow L, Falck Winther J, Glattre E, Olafsdottir G, et al. Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: a population-based study in the Nordic countries. J Clin Oncol. 2001;19(13):3173–3181. - PubMed
-
- Cardous-Ubbink MC, Heinen RC, Langeveld NE, Bakker PJ, Voute PA, Caron HN, et al. Long-term cause-specific mortality among five-year survivors of childhood cancer. Pediatr Blood Cancer. 2004;42(7):563–573. - PubMed
-
- MacArthur AC, Spinelli JJ, Rogers PC, Goddard KJ, Abanto ZU, McBride ML. Mortality among 5-year survivors of cancer diagnosed during childhood or adolescence in British Columbia, Canada. Pediatr Blood Cancer. 2007;48(4):460–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
