Factors influencing attrition in a multisite, randomized, clinical trial following traumatic brain injury in adolescence
- PMID: 24842589
- PMCID: PMC4465204
- DOI: 10.1097/HTR.0000000000000059
Factors influencing attrition in a multisite, randomized, clinical trial following traumatic brain injury in adolescence
Abstract
Background: Attrition in longitudinal research negatively affects statistical power, disrupts statistical stability, and can produce unwanted bias.
Objective: To investigate factors associated with shorter length of study participation and lower rates of study completion (ie, attrition) in a large, multisite, longitudinal, randomized, clinical trial examining the efficacy of a Web-based family problem-solving treatment following traumatic brain injury (TBI) in adolescence.
Setting: Five major trauma centers in the central and western regions of the United States.
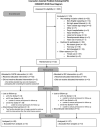
Participants: Children (N = 132) aged 12 to 17 years hospitalized for complicated mild to severe TBI within the previous 6 months.
Results: Completers had a higher primary caregiver education and higher family income than noncompleters, whereas ethnicity, latency to baseline assessment, and intervention group were not significantly associated with study completion.
Conclusion: This is the first study that has specifically examined factors of attrition in a pediatric TBI population. The results suggest that research on pediatric TBI populations may be biased toward higher-income families and highlights the importance of designing studies with increased awareness of the impact of participant demographic factors.
Figures
References
-
- Bender BG, Iklé DN, DuHamel T, Tinkelman D. Retention of asthmatic patients in a longitudinal clinical trial. J Allergy Clin Immunol. 1997;99(2):197–203. - PubMed
-
- Carroll LJ, Cassidy JD, Holm L, Kraus J, Coronado VG. Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;36:113–125. - PubMed
-
- Harris PM. Attrition revisited. Am J Eval. 1998;19(3):293–305.
-
- Cicchetti DV, Nelson LD. Re-examining threats to the reliability and validity of putative brain-behavior relationships: new guidelines for assessing the effect of patients lost to follow-up. J Clin Exp Neuropsychol. 1994;16(3):339–343. - PubMed
-
- Karlson CW, Rapoff MA. Attrition in randomized controlled trials for pediatric chronic conditions. J Pediatr Psychol. 2009;34(7):782–793. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources