Apple derived cellulose scaffolds for 3D mammalian cell culture
- PMID: 24842603
- PMCID: PMC4026483
- DOI: 10.1371/journal.pone.0097835
Apple derived cellulose scaffolds for 3D mammalian cell culture
Abstract
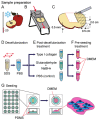
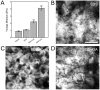
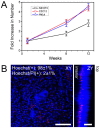
There are numerous approaches for producing natural and synthetic 3D scaffolds that support the proliferation of mammalian cells. 3D scaffolds better represent the natural cellular microenvironment and have many potential applications in vitro and in vivo. Here, we demonstrate that 3D cellulose scaffolds produced by decellularizing apple hypanthium tissue can be employed for in vitro 3D culture of NIH3T3 fibroblasts, mouse C2C12 muscle myoblasts and human HeLa epithelial cells. We show that these cells can adhere, invade and proliferate in the cellulose scaffolds. In addition, biochemical functionalization or chemical cross-linking can be employed to control the surface biochemistry and/or mechanical properties of the scaffold. The cells retain high viability even after 12 continuous weeks of culture and can achieve cell densities comparable with other natural and synthetic scaffold materials. Apple derived cellulose scaffolds are easily produced, inexpensive and originate from a renewable source. Taken together, these results demonstrate that naturally derived cellulose scaffolds offer a complementary approach to existing techniques for the in vitro culture of mammalian cells in a 3D environment.
Conflict of interest statement
Figures



Similar articles
-
Decellularized Apple-Derived Scaffolds for Bone Tissue Engineering In Vitro and In Vivo.J Vis Exp. 2024 Feb 23;(204). doi: 10.3791/65226. J Vis Exp. 2024. PMID: 38465930
-
Novel synthesis strategies for natural polymer and composite biomaterials as potential scaffolds for tissue engineering.Philos Trans A Math Phys Eng Sci. 2010 Apr 28;368(1917):1981-97. doi: 10.1098/rsta.2010.0009. Philos Trans A Math Phys Eng Sci. 2010. PMID: 20308112 Free PMC article.
-
Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture.J Control Release. 2012 Dec 28;164(3):291-8. doi: 10.1016/j.jconrel.2012.06.039. Epub 2012 Jul 7. J Control Release. 2012. PMID: 22776290
-
Three-Dimensional Scaffolds for Intestinal Cell Culture: Fabrication, Utilization, and Prospects.Tissue Eng Part B Rev. 2024 Apr;30(2):158-175. doi: 10.1089/ten.TEB.2023.0124. Epub 2023 Sep 22. Tissue Eng Part B Rev. 2024. PMID: 37646409 Review.
-
Scaffolds for tissue engineering and 3D cell culture.Methods Mol Biol. 2011;695:17-39. doi: 10.1007/978-1-60761-984-0_2. Methods Mol Biol. 2011. PMID: 21042963 Review.
Cited by
-
Plant Decellularization by Chemical and Physical Methods for Regenerative Medicine: A Review Article.J Med Signals Sens. 2024 Apr 18;14:10. doi: 10.4103/jmss.jmss_20_22. eCollection 2024. J Med Signals Sens. 2024. PMID: 38993202 Free PMC article. Review.
-
Natural Architectures for Tissue Engineering and Regenerative Medicine.J Funct Biomater. 2020 Jul 7;11(3):47. doi: 10.3390/jfb11030047. J Funct Biomater. 2020. PMID: 32645945 Free PMC article. Review.
-
Biocompatibility of Subcutaneously Implanted Plant-Derived Cellulose Biomaterials.PLoS One. 2016 Jun 21;11(6):e0157894. doi: 10.1371/journal.pone.0157894. eCollection 2016. PLoS One. 2016. PMID: 27328066 Free PMC article.
-
Plant-Derived Nanocellulose as Structural and Mechanical Reinforcement of Freeze-Cast Chitosan Scaffolds for Biomedical Applications.Biomacromolecules. 2019 Oct 14;20(10):3733-3745. doi: 10.1021/acs.biomac.9b00784. Epub 2019 Sep 26. Biomacromolecules. 2019. PMID: 31454234 Free PMC article.
-
Recent Advances in Modified Cellulose for Tissue Culture Applications.Molecules. 2018 Mar 14;23(3):654. doi: 10.3390/molecules23030654. Molecules. 2018. PMID: 29538287 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

