Nanoclay-enriched poly(ɛ-caprolactone) electrospun scaffolds for osteogenic differentiation of human mesenchymal stem cells
- PMID: 24842693
- PMCID: PMC4137355
- DOI: 10.1089/ten.tea.2013.0281
Nanoclay-enriched poly(ɛ-caprolactone) electrospun scaffolds for osteogenic differentiation of human mesenchymal stem cells
Abstract
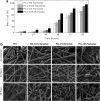
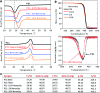
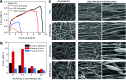
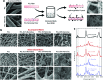
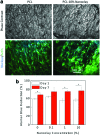
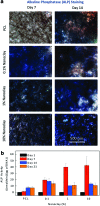
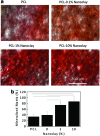
Musculoskeletal tissue engineering aims at repairing and regenerating damaged tissues using biological tissue substitutes. One approach to achieve this aim is to develop osteoconductive scaffolds that facilitate the formation of functional bone tissue. We have fabricated nanoclay-enriched electrospun poly(ɛ-caprolactone) (PCL) scaffolds for osteogenic differentiation of human mesenchymal stem cells (hMSCs). A range of electrospun scaffolds is fabricated by varying the nanoclay concentrations within the PCL scaffolds. The addition of nanoclay decreases fiber diameter and increases surface roughness of electrospun fibers. The enrichment of PCL scaffold with nanoclay promotes in vitro biomineralization when subjected to simulated body fluid (SBF), indicating bioactive characteristics of the hybrid scaffolds. The degradation rate of PCL increases due to the addition of nanoclay. In addition, a significant increase in crystallization temperature of PCL is also observed due to enhanced surface interactions between PCL and nanoclay. The effect of nanoclay on the mechanical properties of electrospun fibers is also evaluated. The feasibility of using nanoclay-enriched PCL scaffolds for tissue engineering applications is investigated in vitro using hMSCs. The nanoclay-enriched electrospun PCL scaffolds support hMSCs adhesion and proliferation. The addition of nanoclay significantly enhances osteogenic differentiation of hMSCs on the electrospun scaffolds as evident by an increase in alkaline phosphates activity of hMSCs and higher deposition of mineralized extracellular matrix compared to PCL scaffolds. Given its unique bioactive characteristics, nanoclay-enriched PCL fibrous scaffold may be used for musculoskeletal tissue engineering.
Figures

Similar articles
-
Role of nanofibrous poly(caprolactone) scaffolds in human mesenchymal stem cell attachment and spreading for in vitro bone tissue engineering--response to osteogenic regulators.Tissue Eng Part A. 2010 Feb;16(2):393-404. doi: 10.1089/ten.TEA.2009.0242. Tissue Eng Part A. 2010. PMID: 19772455
-
Embedded silica nanoparticles in poly(caprolactone) nanofibrous scaffolds enhanced osteogenic potential for bone tissue engineering.Tissue Eng Part A. 2012 Sep;18(17-18):1867-81. doi: 10.1089/ten.TEA.2012.0167. Tissue Eng Part A. 2012. Retraction in: Tissue Eng Part A. 2019 Apr;25(7-8):677. doi: 10.1089/ten.tea.2012.0167.retract. PMID: 22725098 Retracted.
-
Biomineralized hydroxyapatite nanoclay composite scaffolds with polycaprolactone for stem cell-based bone tissue engineering.J Biomed Mater Res A. 2015 Jun;103(6):2077-101. doi: 10.1002/jbm.a.35342. Epub 2014 Oct 21. J Biomed Mater Res A. 2015. PMID: 25331212
-
Advances in Electrospun Poly(ε-caprolactone)-Based Nanofibrous Scaffolds for Tissue Engineering.Polymers (Basel). 2024 Oct 10;16(20):2853. doi: 10.3390/polym16202853. Polymers (Basel). 2024. PMID: 39458681 Free PMC article. Review.
-
Applications of Poly(caprolactone)-based Nanofibre Electrospun Scaffolds in Tissue Engineering and Regenerative Medicine.Curr Stem Cell Res Ther. 2021;16(4):414-442. doi: 10.2174/1574888X15666201014145703. Curr Stem Cell Res Ther. 2021. PMID: 33059569 Review.
Cited by
-
Evaluation of osteoconductive effect of polycaprolactone (PCL) scaffold treated with Aloe vera on adipose-derived mesenchymal stem cells (ADSCs).Am J Stem Cells. 2023 Oct 20;12(4):83-91. eCollection 2023. Am J Stem Cells. 2023. PMID: 38021455 Free PMC article.
-
Preparation and Characterization of Nano-Laponite/PLGA Composite Scaffolds for Urethra Tissue Engineering.Mol Biotechnol. 2020 Mar;62(3):192-199. doi: 10.1007/s12033-020-00237-z. Mol Biotechnol. 2020. PMID: 32016781
-
Three-dimensional porous poly(propylene fumarate)-co-poly(lactic-co-glycolic acid) scaffolds for tissue engineering.J Biomed Mater Res A. 2018 Sep;106(9):2507-2517. doi: 10.1002/jbm.a.36446. J Biomed Mater Res A. 2018. PMID: 29707898 Free PMC article.
-
Nano-Silicate-Reinforced and SDF-1α-Loaded Gelatin-Methacryloyl Hydrogel for Bone Tissue Engineering.Int J Nanomedicine. 2020 Nov 24;15:9337-9353. doi: 10.2147/IJN.S270681. eCollection 2020. Int J Nanomedicine. 2020. Retraction in: Int J Nanomedicine. 2025 Feb 21;20:2325-2326. doi: 10.2147/IJN.S523935. PMID: 33262591 Free PMC article. Retracted.
-
Fabrication and Evaluation of Layered Double Hydroxide-Enriched ß-Tricalcium Phosphate Nanocomposite Granules for Bone Regeneration: In Vitro Study.Mol Biotechnol. 2021 Jun;63(6):477-490. doi: 10.1007/s12033-021-00315-w. Epub 2021 Mar 23. Mol Biotechnol. 2021. PMID: 33755861
References
-
- Khademhosseini A., Vacanti J., and Langer R.Progress in tissue engineering. Sci Am Mag 300,64, 2009 - PubMed
-
- Peppas N.A., Hilt J.Z., Khademhosseini A., and Langer R.Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater 18,1345, 2006
-
- Yang S., Leong K.-F., Du Z., and Chua C.-K.The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng 7,679, 2001 - PubMed
-
- Detamore M.S., and Athanasiou K.A.Motivation, characterization, and strategy for tissue engineering the temporomandibular joint disc. Tissue Eng 9,1065, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

