Tumor-Absorbed Dose Predicts Progression-Free Survival Following (131)I-Tositumomab Radioimmunotherapy
- PMID: 24842891
- PMCID: PMC4237694
- DOI: 10.2967/jnumed.113.136044
Tumor-Absorbed Dose Predicts Progression-Free Survival Following (131)I-Tositumomab Radioimmunotherapy
Abstract
The study aimed at identifying patient-specific dosimetric and nondosimetric factors predicting outcome of non-Hodgkin lymphoma patients after (131)I-tositumomab radioimmunotherapy for potential use in treatment planning.
Methods: Tumor-absorbed dose measures were estimated for 130 tumors in 39 relapsed or refractory non-Hodgkin lymphoma patients by coupling SPECT/CT imaging with the Dose Planning Method (DPM) Monte Carlo code. Equivalent biologic effect was calculated to assess the biologic effects of nonuniform absorbed dose including the effects of the unlabeled antibody. Evaluated nondosimetric covariates included histology, presence of bulky disease, and prior treatment history. Tumor level outcome was based on volume shrinkage assessed on follow-up CT. Patient level outcome measures were overall response (OR), complete response (CR), and progression-free survival (PFS), determined from clinical assessments that included PET/CT.
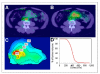
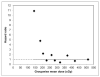
Results: The estimated mean tumor-absorbed dose had a median value of 275 cGy (range, 94-711 cGy). A high correlation was observed between tracer-predicted and therapy-delivered mean tumor-absorbed doses (P < 0.001; r = 0.85). In univariate tumor-level analysis, tumor shrinkage correlated significantly with almost all of the evaluated dosimetric factors, including equivalent biologic effect. Regression analysis showed that OR, CR, and PFS were associated with the dosimetric factors and equivalent biologic effect. Both mean tumor-absorbed dose (P = 0.025) and equivalent biologic effect (P = 0.035) were significant predictors of PFS whereas none of the nondosimetric covariates were found to be statistically significant factors affecting PFS. The most important finding of the study was that in Kaplan-Meier curves stratified by mean dose, longer PFS was observed in patients receiving mean tumor-absorbed doses greater than 200 cGy than in those receiving 200 cGy or less (median PFS, 13.6 vs. 1.9 mo for the 2 dose groups; log-rank P < 0.0001).
Conclusion: A higher mean tumor-absorbed dose was significantly predictive of improved PFS after (131)I-tositumomab radioimmunotherapy. Hence tumor-absorbed dose, which can be estimated before therapy, can potentially be used to design radioimmunotherapy protocols to improve efficacy.
Keywords: SPECT/CT; dosimetry; non-Hodgkin lymphoma; progression free survival; radioimmunotherapy.
© 2014 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
Figures


Similar articles
-
131I-tositumomab radioimmunotherapy: initial tumor dose-response results using 3-dimensional dosimetry including radiobiologic modeling.J Nucl Med. 2010 Jul;51(7):1155-62. doi: 10.2967/jnumed.110.075176. Epub 2010 Jun 16. J Nucl Med. 2010. PMID: 20554734 Free PMC article.
-
Patient-specific, 3-dimensional dosimetry in non-Hodgkin's lymphoma patients treated with 131I-anti-B1 antibody: assessment of tumor dose-response.J Nucl Med. 2003 Feb;44(2):260-8. J Nucl Med. 2003. PMID: 12571219 Clinical Trial.
-
Update on hybrid conjugate-view SPECT tumor dosimetry and response in 131I-tositumomab therapy of previously untreated lymphoma patients.J Nucl Med. 2003 Mar;44(3):457-64. J Nucl Med. 2003. PMID: 12621015
-
Radioimmunotherapy dosimetry--a review.Acta Oncol. 1993;32(7-8):807-17. doi: 10.3109/02841869309096140. Acta Oncol. 1993. PMID: 8305230 Review.
-
The clinical importance of dosimetry in radioimmunotherapy with tositumomab and iodine I 131 tositumomab.Semin Oncol. 2003 Apr;30(2 Suppl 4):31-8. doi: 10.1053/sonc.2003.23799. Semin Oncol. 2003. PMID: 12728405 Review.
Cited by
-
A no-gold-standard technique for objective assessment of quantitative nuclear-medicine imaging methods.Phys Med Biol. 2016 Apr 7;61(7):2780-800. doi: 10.1088/0031-9155/61/7/2780. Epub 2016 Mar 16. Phys Med Biol. 2016. PMID: 26982626 Free PMC article.
-
Dosimetry in Clinical Radiopharmaceutical Therapy of Cancer: Practicality Versus Perfection in Current Practice.J Nucl Med. 2021 Dec;62(Suppl 3):60S-72S. doi: 10.2967/jnumed.121.262977. J Nucl Med. 2021. PMID: 34857623 Free PMC article. Review.
-
FDG PET/CT and Dosimetric Studies of 177Lu-Lilotomab Satetraxetan in a First-in-Human Trial for Relapsed Indolent non-Hodgkin Lymphoma-Are We Hitting the Target?Mol Imaging Biol. 2022 Oct;24(5):807-817. doi: 10.1007/s11307-022-01731-3. Epub 2022 Apr 29. Mol Imaging Biol. 2022. PMID: 35486292 Free PMC article. Clinical Trial.
-
Variations in the practice of molecular radiotherapy and implementation of dosimetry: results from a European survey.EJNMMI Phys. 2017 Dec 4;4(1):28. doi: 10.1186/s40658-017-0193-4. EJNMMI Phys. 2017. PMID: 29199391 Free PMC article.
-
Radiopharmaceutical therapy in cancer: clinical advances and challenges.Nat Rev Drug Discov. 2020 Sep;19(9):589-608. doi: 10.1038/s41573-020-0073-9. Epub 2020 Jul 29. Nat Rev Drug Discov. 2020. PMID: 32728208 Free PMC article. Review.
References
-
- Jacene HA, Filice R, Kasecamp W, Wahl RL. Comparison of 90Y-ibritumomab tiuxetan and 131I-tositumomab in clinical practice. J Nucl Med. 2007;48:1767–1776. - PubMed
-
- Kaminski MS, Tuck M, Estes J, et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med. 2005;352:441–449. - PubMed
-
- Sgouros G, Squeri S, Ballangrud AM, et al. Patient-specific, 3-dimensional dosimetry in non-Hodgkin’s lymphoma patients treated with 131I-anti-B1 antibody: assessment of tumor dose-response. J Nucl Med. 2003;44:260–268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
