Effect of maternal cardiovascular conditions and risk factors on offspring cardiovascular disease
- PMID: 24842934
- PMCID: PMC4053195
- DOI: 10.1161/CIRCULATIONAHA.113.001805
Effect of maternal cardiovascular conditions and risk factors on offspring cardiovascular disease
Keywords: cardiovascular diseases; embryonic and fetal development; fetal development; immunity; pregnancy; risk factors.
Conflict of interest statement
Figures
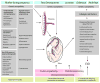
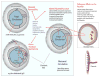
References
-
- Oyen N, Poulsen G, Boyd HA, Wohlfahrt J, Jensen PK, Melbye M. Recurrence of congenital heart defects in families. Circulation. 2009;120:295–301. - PubMed
-
- Ullmo S, Vial Y, Di BS, Roth-Kleiner M, Mivelaz Y, Sekarski N, Ruiz J, Meijboom EJ. Pathologic ventricular hypertrophy in the offspring of diabetic mothers: a retrospective study. Eur Heart J. 2007;28:1319–1325. - PubMed
-
- Cedergren MI, Kallen BA. Maternal obesity and infant heart defects. Obes Res. 2003;11:1065–1071. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

