Epidermal barrier defects link atopic dermatitis with altered skin cancer susceptibility
- PMID: 24843010
- PMCID: PMC4007207
- DOI: 10.7554/eLife.01888
Epidermal barrier defects link atopic dermatitis with altered skin cancer susceptibility
Abstract
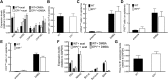
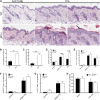
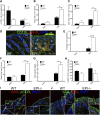
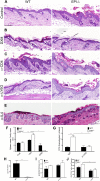
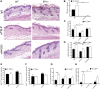
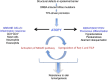
Atopic dermatitis can result from loss of structural proteins in the outermost epidermal layers, leading to a defective epidermal barrier. To test whether this influences tumour formation, we chemically induced tumours in EPI-/- mice, which lack three barrier proteins-Envoplakin, Periplakin, and Involucrin. EPI-/- mice were highly resistant to developing benign tumours when treated with 7,12-dimethylbenz(a)anthracene (DMBA) and 12-O-tetradecanoylphorbol-13-acetate (TPA). The DMBA response was normal, but EPI-/- skin exhibited an exaggerated atopic response to TPA, characterised by abnormal epidermal differentiation, a complex immune infiltrate and elevated serum thymic stromal lymphopoietin (TSLP). The exacerbated TPA response could be normalised by blocking TSLP or the immunoreceptor NKG2D but not CD4+ T cells. We conclude that atopy is protective against skin cancer in our experimental model and that the mechanism involves keratinocytes communicating with cells of the immune system via signalling elements that normally protect against environmental assaults.DOI: http://dx.doi.org/10.7554/eLife.01888.001.
Keywords: cancer; eczema; skin.
Copyright © 2014, Cipolat et al.
Conflict of interest statement
FMW: Deputy Editor,
The other authors declare that no competing interests exist.
Figures



References
-
- Aberg KM, Man MQ, Gallo RL, Ganz T, Crumrine D, Brown BE, Choi EH, Kim DK, Schroder JM, Feingold KR, Elias PM. 2008. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. The Journal of Investigative Dermatology 128:917–925. doi: 10.1038/sj.jid.5701099 - DOI - PMC - PubMed
-
- Aho S, Li K, Ryoo Y, McGee C, Ishida-Yamamoto A, Uitto J, Klement JF. 2004. Periplakin gene targeting reveals a constituent of the cornified cell envelope dispensable for normal mouse development. Molecular and Cellular Biology 24:6410–6418. doi: 10.1128/MCB.24.14.6410-6418.2004 - DOI - PMC - PubMed
-
- Antsiferova M, Huber M, Meyer M, Piwko-Czuchra A, Ramadan T, MacLeod AS, Havran WL, Dummer R, Hohl D, Werner S. 2011. Activin enhances skin tumourigenesis and malignant progression by inducing a pro-tumourigenic immune cell response. Nature Communications 2:576. doi: 10.1038/ncomms1585 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

