Golgi and plasma membrane pools of PI(4)P contribute to plasma membrane PI(4,5)P2 and maintenance of KCNQ2/3 ion channel current
- PMID: 24843134
- PMCID: PMC4050574
- DOI: 10.1073/pnas.1407133111
Golgi and plasma membrane pools of PI(4)P contribute to plasma membrane PI(4,5)P2 and maintenance of KCNQ2/3 ion channel current
Abstract
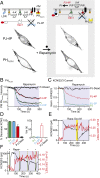
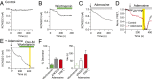
Plasma membrane (PM) phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] regulates the activity of many ion channels and other membrane-associated proteins. To determine precursor sources of the PM PI(4,5)P2 pool in tsA-201 cells, we monitored KCNQ2/3 channel currents and translocation of PHPLCδ1 domains as real-time indicators of PM PI(4,5)P2, and translocation of PHOSH2×2, and PHOSH1 domains as indicators of PM and Golgi phosphatidylinositol 4-phosphate [PI(4)P], respectively. We selectively depleted PI(4)P pools at the PM, Golgi, or both using the rapamycin-recruitable lipid 4-phosphatases. Depleting PI(4)P at the PM with a recruitable 4-phosphatase (Sac1) results in a decrease of PI(4,5)P2 measured by electrical or optical indicators. Depleting PI(4)P at the Golgi with the 4-phosphatase or disrupting membrane-transporting motors induces a decline in PM PI(4,5)P2. Depleting PI(4)P simultaneously at both the Golgi and the PM induces a larger decrease of PI(4,5)P2. The decline of PI(4,5)P2 following 4-phosphatase recruitment takes 1-2 min. Recruiting the endoplasmic reticulum (ER) toward the Golgi membranes mimics the effects of depleting PI(4)P at the Golgi, apparently due to the trans actions of endogenous ER Sac1. Thus, maintenance of the PM pool of PI(4,5)P2 appears to depend on precursor pools of PI(4)P both in the PM and in the Golgi. The decrease in PM PI(4,5)P2 when Sac1 is recruited to the Golgi suggests that the Golgi contribution is ongoing and that PI(4,5)P2 production may be coupled to important cell biological processes such as membrane trafficking or lipid transfer activity.
Keywords: phosphoinositides; pleckstrin homology domain; wortmannin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Optogenetic control of phosphoinositide metabolism.Proc Natl Acad Sci U S A. 2012 Aug 28;109(35):E2316-23. doi: 10.1073/pnas.1211305109. Epub 2012 Jul 30. Proc Natl Acad Sci U S A. 2012. PMID: 22847441 Free PMC article.
-
RdgBα reciprocally transfers PA and PI at ER-PM contact sites to maintain PI(4,5)P2 homoeostasis during phospholipase C signalling in Drosophila photoreceptors.Biochem Soc Trans. 2016 Feb;44(1):286-92. doi: 10.1042/BST20150228. Biochem Soc Trans. 2016. PMID: 26862217 Review.
-
PI(4,5)P2 controls plasma membrane PI4P and PS levels via ORP5/8 recruitment to ER-PM contact sites.J Cell Biol. 2018 May 7;217(5):1797-1813. doi: 10.1083/jcb.201710095. Epub 2018 Feb 22. J Cell Biol. 2018. PMID: 29472386 Free PMC article.
-
Osmoregulatory inositol transporter SMIT1 modulates electrical activity by adjusting PI(4,5)P2 levels.Proc Natl Acad Sci U S A. 2016 Jun 7;113(23):E3290-9. doi: 10.1073/pnas.1606348113. Epub 2016 May 23. Proc Natl Acad Sci U S A. 2016. PMID: 27217553 Free PMC article.
-
Regulation of yeast polarized exocytosis by phosphoinositide lipids.Cell Mol Life Sci. 2024 Nov 19;81(1):457. doi: 10.1007/s00018-024-05483-x. Cell Mol Life Sci. 2024. PMID: 39560727 Free PMC article. Review.
Cited by
-
Plasticity of PI4KIIIα interactions at the plasma membrane.EMBO Rep. 2015 Mar;16(3):312-20. doi: 10.15252/embr.201439151. Epub 2015 Jan 21. EMBO Rep. 2015. PMID: 25608530 Free PMC article.
-
Understanding phosphoinositides: rare, dynamic, and essential membrane phospholipids.Biochem J. 2019 Jan 7;476(1):1-23. doi: 10.1042/BCJ20180022. Biochem J. 2019. PMID: 30617162 Free PMC article. Review.
-
Phosphoinositide transport and metabolism at membrane contact sites.Biochim Biophys Acta Mol Cell Biol Lipids. 2022 Mar;1867(3):159107. doi: 10.1016/j.bbalip.2021.159107. Epub 2022 Jan 4. Biochim Biophys Acta Mol Cell Biol Lipids. 2022. PMID: 34995791 Free PMC article. Review.
-
Triggered Golgi membrane enrichment promotes PtdIns(4,5)P2 generation for plasma membrane repair.J Cell Biol. 2023 Aug 7;222(8):e202303017. doi: 10.1083/jcb.202303017. Epub 2023 May 9. J Cell Biol. 2023. PMID: 37158801 Free PMC article.
-
BRET-monitoring of the dynamic changes of inositol lipid pools in living cells reveals a PKC-dependent PtdIns4P increase upon EGF and M3 receptor activation.Biochim Biophys Acta. 2016 Mar;1861(3):177-87. doi: 10.1016/j.bbalip.2015.12.005. Epub 2015 Dec 12. Biochim Biophys Acta. 2016. PMID: 26692031 Free PMC article.
References
-
- Doughman RL, Firestone AJ, Anderson RA. Phosphatidylinositol phosphate kinases put PI4,5P2 in its place. J Membr Biol. 2003;194(2):77–89. - PubMed
-
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443(7112):651–657. - PubMed
-
- Zhao X, et al. Interaction of neuronal calcium sensor-1 (NCS-1) with phosphatidylinositol 4-kinase β stimulates lipid kinase activity and affects membrane trafficking in COS-7 cells. J Biol Chem. 2001;276(43):40183–40189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

