Promotion of atomic hydrogen recombination as an alternative to electron trapping for the role of metals in the photocatalytic production of H2
- PMID: 24843154
- PMCID: PMC4050604
- DOI: 10.1073/pnas.1405365111
Promotion of atomic hydrogen recombination as an alternative to electron trapping for the role of metals in the photocatalytic production of H2
Abstract
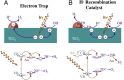
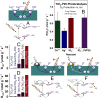
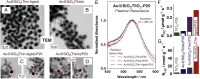
The production of hydrogen from water with semiconductor photocatalysts can be promoted by adding small amounts of metals to their surfaces. The resulting enhancement in photocatalytic activity is commonly attributed to a fast transfer of the excited electrons generated by photon absorption from the semiconductor to the metal, a step that prevents deexcitation back to the ground electronic state. Here we provide experimental evidence that suggests an alternative pathway that does not involve electron transfer to the metal but requires it to act as a catalyst for the recombination of the hydrogen atoms made via the reduction of protons on the surface of the semiconductor instead.
Keywords: core-shell nanostructures; gold–titania; hydrogen production; photocatalysis; time-resolved fluorescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Palmisano G, Augugliaro V, Pagliaro M, Palmisano L. Photocatalysis: A promising route for 21st century organic chemistry. Chem Commun (Camb) 2007;(33):3425–3437. - PubMed
-
- Kabra K, Chaudhary R, Sawhney RL. Treatment of hazardous organic and inorganic compounds through aqueous-phase photocatalysis: A review. Ind Eng Chem Res. 2004;43(24):7683–7696.
-
- Bahnemann D. Photocatalytic water treatment: Solar energy applications. Sol Energy. 2004;77(5):445–459.
-
- Kamat PV. Meeting the clean energy demand: Nanostructure architectures for solar energy conversion. J Phys Chem C. 2007;111(7):2834–2860.
-
- Ni M, Leung MKH, Leung DYC, Sumathy K. A review and recent developments in photocatalytic water-splitting using for hydrogen production. Renew Sustain Energy Rev. 2007;11(3):401–425.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

