Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2 fixation
- PMID: 24843170
- PMCID: PMC4050595
- DOI: 10.1073/pnas.1402028111
Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2 fixation
Abstract
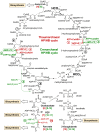
Archaea of the phylum Thaumarchaeota are among the most abundant prokaryotes on Earth and are widely distributed in marine, terrestrial, and geothermal environments. All studied Thaumarchaeota couple the oxidation of ammonia at extremely low concentrations with carbon fixation. As the predominant nitrifiers in the ocean and in various soils, ammonia-oxidizing archaea contribute significantly to the global nitrogen and carbon cycles. Here we provide biochemical evidence that thaumarchaeal ammonia oxidizers assimilate inorganic carbon via a modified version of the autotrophic hydroxypropionate/hydroxybutyrate cycle of Crenarchaeota that is far more energy efficient than any other aerobic autotrophic pathway. The identified genes of this cycle were found in the genomes of all sequenced representatives of the phylum Thaumarchaeota, indicating the environmental significance of this efficient CO2-fixation pathway. Comparative phylogenetic analysis of proteins of this pathway suggests that the hydroxypropionate/hydroxybutyrate cycle emerged independently in Crenarchaeota and Thaumarchaeota, thus supporting the hypothesis of an early evolutionary separation of both archaeal phyla. We conclude that high efficiency of anabolism exemplified by this autotrophic cycle perfectly suits the lifestyle of ammonia-oxidizing archaea, which thrive at a constantly low energy supply, thus offering a biochemical explanation for their ecological success in nutrient-limited environments.
Keywords: Nitrosopumilus maritimus; autotrophy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Convergent Evolution of a Promiscuous 3-Hydroxypropionyl-CoA Dehydratase/Crotonyl-CoA Hydratase in Crenarchaeota and Thaumarchaeota.mSphere. 2021 Jan 20;6(1):e01079-20. doi: 10.1128/mSphere.01079-20. mSphere. 2021. PMID: 33472982 Free PMC article.
-
Malonic semialdehyde reductase from the archaeon Nitrosopumilus maritimus is involved in the autotrophic 3-hydroxypropionate/4-hydroxybutyrate cycle.Appl Environ Microbiol. 2015 Mar;81(5):1700-7. doi: 10.1128/AEM.03390-14. Epub 2014 Dec 29. Appl Environ Microbiol. 2015. PMID: 25548047 Free PMC article.
-
Autotrophic ammonia oxidation by soil thaumarchaea.Proc Natl Acad Sci U S A. 2010 Oct 5;107(40):17240-5. doi: 10.1073/pnas.1004947107. Epub 2010 Sep 20. Proc Natl Acad Sci U S A. 2010. PMID: 20855593 Free PMC article.
-
The Thaumarchaeota: an emerging view of their phylogeny and ecophysiology.Curr Opin Microbiol. 2011 Jun;14(3):300-6. doi: 10.1016/j.mib.2011.04.007. Epub 2011 May 4. Curr Opin Microbiol. 2011. PMID: 21546306 Free PMC article. Review.
-
Beyond the Calvin cycle: autotrophic carbon fixation in the ocean.Ann Rev Mar Sci. 2011;3:261-89. doi: 10.1146/annurev-marine-120709-142712. Ann Rev Mar Sci. 2011. PMID: 21329206 Review.
Cited by
-
Transcriptomic Insights into Archaeal Nitrification in the Amundsen Sea Polynya, Antarctica.J Microbiol. 2023 Nov;61(11):967-980. doi: 10.1007/s12275-023-00090-0. Epub 2023 Dec 7. J Microbiol. 2023. PMID: 38062325
-
Leave no stone unturned: individually adapted xerotolerant Thaumarchaeota sheltered below the boulders of the Atacama Desert hyperarid core.Microbiome. 2021 Nov 26;9(1):234. doi: 10.1186/s40168-021-01177-9. Microbiome. 2021. PMID: 34836555 Free PMC article.
-
Autotrophic carbon fixation pathways along the redox gradient in oxygen-depleted oceanic waters.Environ Microbiol Rep. 2020 Jun;12(3):334-341. doi: 10.1111/1758-2229.12837. Epub 2020 Apr 7. Environ Microbiol Rep. 2020. PMID: 32202395 Free PMC article.
-
Archaeal Diversity and CO2 Fixers in Carbonate-/Siliciclastic-Rock Groundwater Ecosystems.Archaea. 2017 Jun 13;2017:2136287. doi: 10.1155/2017/2136287. eCollection 2017. Archaea. 2017. PMID: 28694737 Free PMC article.
-
Integration of Metagenomic and Stable Carbon Isotope Evidence Reveals the Extent and Mechanisms of Carbon Dioxide Fixation in High-Temperature Microbial Communities.Front Microbiol. 2017 Feb 3;8:88. doi: 10.3389/fmicb.2017.00088. eCollection 2017. Front Microbiol. 2017. PMID: 28217111 Free PMC article.
References
-
- Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P. Mesophilic Crenarchaeota: Proposal for a third archaeal phylum, the Thaumarchaeota. Nat Rev Microbiol. 2008;6(3):245–252. - PubMed
-
- Könneke M, et al. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature. 2005;437(7058):543–546. - PubMed
-
- Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature. 2009;461(7266):976–979. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

