Fluid shear stress threshold regulates angiogenic sprouting
- PMID: 24843171
- PMCID: PMC4050561
- DOI: 10.1073/pnas.1310842111
Fluid shear stress threshold regulates angiogenic sprouting
Abstract
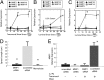
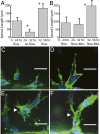
The density and architecture of capillary beds that form within a tissue depend on many factors, including local metabolic demand and blood flow. Here, using microfluidic control of local fluid mechanics, we show the existence of a previously unappreciated flow-induced shear stress threshold that triggers angiogenic sprouting. Both intraluminal shear stress over the endothelium and transmural flow through the endothelium above 10 dyn/cm(2) triggered endothelial cells to sprout and invade into the underlying matrix, and this threshold is not impacted by the maturation of cell-cell junctions or pressure gradient across the monolayer. Antagonizing VE-cadherin widened cell-cell junctions and reduced the applied shear stress for a given transmural flow rate, but did not affect the shear threshold for sprouting. Furthermore, both transmural and luminal flow induced expression of matrix metalloproteinase 1, and this up-regulation was required for the flow-induced sprouting. Once sprouting was initiated, continuous flow was needed to both sustain sprouting and prevent retraction. To explore the potential ramifications of a shear threshold on the spatial patterning of new sprouts, we used finite-element modeling to predict fluid shear in a variety of geometric settings and then experimentally demonstrated that transmural flow guided preferential sprouting toward paths of draining interstitial fluid flow as might occur to connect capillary beds to venules or lymphatics. In addition, we show that luminal shear increases in local narrowings of vessels to trigger sprouting, perhaps ultimately to normalize shear stress across the vasculature. Together, these studies highlight the role of shear stress in controlling angiogenic sprouting and offer a potential homeostatic mechanism for regulating vascular density.
Keywords: angiogenesis; force; mechanotransduction; migration; morphogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–887. - PubMed
-
- Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol. 1990;259(4 Pt 2):H1063–H1070. - PubMed
-
- Dawson JM, Hudlická O. The effects of long term administration of prazosin on the microcirculation in skeletal muscles. Cardiovasc Res. 1989;23(11):913–920. - PubMed
-
- Egginton S, Zhou AL, Brown MD, Hudlická O. Unorthodox angiogenesis in skeletal muscle. Cardiovasc Res. 2001;49(3):634–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

