Development and validation of a HPLC method for the determination of cyclosporine a in new bioadhesive nanoparticles for oral administration
- PMID: 24843186
- PMCID: PMC4023282
Development and validation of a HPLC method for the determination of cyclosporine a in new bioadhesive nanoparticles for oral administration
Abstract
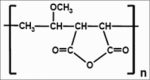
A simple and reliable high performance liquid chromatography method was developed and validated for the rapid determination of cyclosporine A in new pharmaceutical dosage forms based on the use of poly (methylvinylether-co-maleic anhydride) nanoparticles. The chromatographic separation was achieved using Ultrabase C18 column (250×4.6 mm, 5 μm), which was kept at 75°. The gradient mobile phase consisted of acetonitrile and water with a flow rate of 1 ml/min. The effluent was monitored at 205 nm using diode array detector. The method exhibited linearity over the assayed concentration range (22-250 μg/ml) and demonstrated good intraday and interday precision and accuracy (relative standard deviations were less than 6.5% and the deviation from theoretical values is below 5.5%). The detection limit was 1.36 μg/ml. This method was also applied for quantitative analysis of cyclosporine A released from poly (methylvinylether-co-maleic anhydride) nanoparticles.
Keywords: Cyclosporine A; HPLC-UV; nanoparticles; oral administration; poly (methylvinylether-co-maleic anhydride).
Figures



Similar articles
-
Development and Validation of a RP-HPLC Method for Determination of Cyclosporine in Capsule.Indian J Pharm Sci. 2010 Mar;72(2):252-5. doi: 10.4103/0250-474X.65030. Indian J Pharm Sci. 2010. PMID: 20838535 Free PMC article.
-
Determination of Benzalkonium Chloride in a Disinfectant by UV Spectrophotometry and Gas and High-Performance Liquid Chromatography: Validation, Comparison of Characteristics, and Economic Feasibility.Int J Anal Chem. 2022 Sep 19;2022:2932634. doi: 10.1155/2022/2932634. eCollection 2022. Int J Anal Chem. 2022. PMID: 36245786 Free PMC article.
-
Investigation of the Presence of Sildenafil in Herbal Dietary Supplements by Validated HPLC Method.Turk J Pharm Sci. 2020 Feb;17(1):56-62. doi: 10.4274/tjps.galenos.2018.91249. Epub 2020 Feb 19. Turk J Pharm Sci. 2020. PMID: 32454761 Free PMC article.
-
Application of Factorial and Doehlert Designs for the Optimization of the Simultaneous Separation and Determination of Antimigraine Drugs in Pharmaceutical Formulations by RP-HPLC-UV.Int J Anal Chem. 2019 Aug 15;2019:9685750. doi: 10.1155/2019/9685750. eCollection 2019. Int J Anal Chem. 2019. PMID: 31511775 Free PMC article.
-
Simultaneous determination of four neuroprotective compounds of Tilia amurensis by high performance liquid chromatography coupled with diode array detector.Pharmacogn Mag. 2014 Jul;10(39):195-9. doi: 10.4103/0973-1296.137353. Pharmacogn Mag. 2014. PMID: 25210303 Free PMC article.
Cited by
-
Quality by Design (QbD) Based Method for Estimation of Xanthohumol in Bulk and Solid Lipid Nanoparticles and Validation.Molecules. 2023 Jan 4;28(2):472. doi: 10.3390/molecules28020472. Molecules. 2023. PMID: 36677532 Free PMC article.
-
In vitro Characterization and Release Studies of Combined Nonionic Surfactant-Based Vesicles for the Prolonged Delivery of an Immunosuppressant Model Drug.Int J Nanomedicine. 2020 Oct 14;15:7937-7949. doi: 10.2147/IJN.S268846. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33116510 Free PMC article.
-
Quality by Design Based Formulation of Xanthohumol Loaded Solid Lipid Nanoparticles with Improved Bioavailability and Anticancer Effect against PC-3 Cells.Pharmaceutics. 2022 Nov 7;14(11):2403. doi: 10.3390/pharmaceutics14112403. Pharmaceutics. 2022. PMID: 36365221 Free PMC article.
-
Boosting the anti-inflammatory effect of self-assembled hybrid lecithin-chitosan nanoparticles via hybridization with gold nanoparticles for the treatment of psoriasis: elemental mapping and in vivo modeling.Drug Deliv. 2022 Dec;29(1):1726-1742. doi: 10.1080/10717544.2022.2081383. Drug Deliv. 2022. PMID: 35635314 Free PMC article.
-
Analytical quality by design approach to develop an eco-friendly RP-HPLC method for estimation of irbesartan in chitosan polymeric nanoparticles: forced degradation studies and assessment of in vitro release mathematical modelling.RSC Adv. 2024 Jul 12;14(31):22169-22184. doi: 10.1039/d4ra03952a. eCollection 2024 Jul 12. RSC Adv. 2024. PMID: 39005249 Free PMC article.
References
-
- Matzke GR, Luke DR. Dialysis and renal transplant therapy. In: Herfindal ET, Gourley DR, Hart LL, editors. Clinical Pharmacy and Therapeutics. Baltimore: Williams and Wilkins; 1988. pp. 229–42.
-
- Talal N. Cyclosporine as an immunosuppressive agent for autoimmune disease: Theoretical concepts and therapeutic strategies. Transplant Proc. 1988;20:11–5. - PubMed
-
- Hebert MF. Contributions of hepatic and intestinal metabolism and P-glycoprotein to cyclosporine and tacrolimus oral drug delivery. Adv Drug Deliv Rev. 1997;27:201–14. - PubMed
-
- Kolars JC, Awni WM, Merion RM, Watkins PB. First-pass metabolism of cyclosporine by the gut. Lancet. 1991;338:1488–90. - PubMed
LinkOut - more resources
Full Text Sources
