GIP and GLP-1, the two incretin hormones: Similarities and differences
- PMID: 24843404
- PMCID: PMC4020673
- DOI: 10.1111/j.2040-1124.2010.00022.x
GIP and GLP-1, the two incretin hormones: Similarities and differences
Abstract
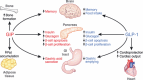
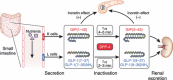
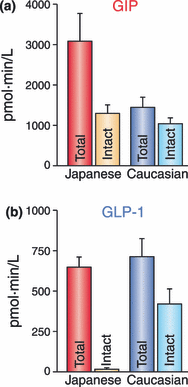
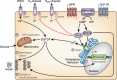
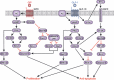
Gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) are the two primary incretin hormones secreted from the intestine on ingestion of glucose or nutrients to stimulate insulin secretion from pancreatic β cells. GIP and GLP-1 exert their effects by binding to their specific receptors, the GIP receptor (GIPR) and the GLP-1 receptor (GLP-1R), which belong to the G-protein coupled receptor family. Receptor binding activates and increases the level of intracellular cyclic adenosine monophosphate in pancreatic β cells, thereby stimulating insulin secretion glucose-dependently. In addition to their insulinotropic effects, GIP and GLP-1 play critical roles in various biological processes in different tissues and organs that express GIPR and GLP-1R, including the pancreas, fat, bone and the brain. Within the pancreas, GIP and GLP-1 together promote β cell proliferation and inhibit apoptosis, thereby expanding pancreatic β cell mass, while GIP enhances postprandial glucagon response and GLP-1 suppresses it. In adipose tissues, GIP but not GLP-1 facilitates fat deposition. In bone, GIP promotes bone formation while GLP-1 inhibits bone absorption. In the brain, both GIP and GLP-1 are thought to be involved in memory formation as well as the control of appetite. In addition to these differences, secretion of GIP and GLP-1 and their insulinotropic effects on β cells have been shown to differ in patients with type 2 diabetes compared to healthy subjects. We summarize here the similarities and differences of these two incretin hormones in secretion and metabolism, their insulinotropic action on pancreatic β cells, and their non-insulinotropic effects, and discuss their potential in treatment of type 2 diabetes. (J Diabetes Invest, doi: 10.1111/j.2040-1124.2010.00022.x, 2010).
Keywords: GIP; GLP‐1; Incretin.
Figures

References
-
- Zunz E, La Barre J. Contributiona a l’etude des variations physiologiques de la secretion interne du pancreas: Relations entre les secretions externe et interne du pancreas. Arch Int Physiol Biochim 1929; 31: 20–44
-
- Elrick H, Stimmler L, Hlad CJ Jr, et al. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab 1964; 24: 1076–1082 - PubMed
-
- McIntyre N, Holdsworth CD, Turner DS. New interpretation of oral glucose tolerance. Lancet 1964; 2: 20–21 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

