28-week, randomized, multicenter, open-label, parallel-group phase III trial to investigate the efficacy and safety of biphasic insulin aspart 70 thrice-daily injections vs twice-daily injections of biphasic insulin aspart 30 in patients with type 2 diabetes
- PMID: 24843416
- PMCID: PMC4008024
- DOI: 10.1111/j.2040-1124.2010.00015.x
28-week, randomized, multicenter, open-label, parallel-group phase III trial to investigate the efficacy and safety of biphasic insulin aspart 70 thrice-daily injections vs twice-daily injections of biphasic insulin aspart 30 in patients with type 2 diabetes
Abstract
Aims/Introduction: An insulin analogue formulation with a 7:3 ratio of rapid-acting and intermediate-acting fractions, biphasic insulin aspart 70 (BIAsp70) was developed to supplement basal insulin between meals and mimic the physiological pattern of postprandial insulin secretion.
Materials and methods: We carried out a randomized, open-label study to compare the efficacy and safety profiles of BIAsp70 and an insulin analogue formulation with a 3:7 ratio of rapid-acting and intermediate-acting fractions (BIAsp30) in type 2 diabetes mellitus patients. Patients were randomized and received either thrice-daily BIAsp70 (n = 145) or twice-daily BIAsp30 (n = 144) for 28 weeks. The primary end-point was glycated hemoglobin (HbA1c) after 16 weeks of treatment.
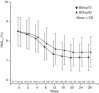
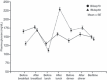
Results: Non-inferiority of BIAsp70 vs BIAsp30 was confirmed and superiority was established with a between-group difference (BIAsp70-BIAsp30) in HbA1c after 16 weeks of treatment of -0.35% (95% CI: -0.51 to -0.19; P < 0.0001 for superiority). The mean postprandial glucose increment (19.96 vs 54.35 mg/dL; P < 0.0001) and M-value (12.99 vs 17.94; P < 0.0001) at 16 weeks were smaller in the BIAsp70 group than in the BIAsp30 group, and were maintained at 28 weeks. Pre-breakfast glucose (157.9 vs 140.7 mg/dL), total insulin dose (46.8 vs 38.1 U/day) and weight gain (+1.94 vs 1.23 kg) at week 28 were greater in the BIAsp70 group. Incidence of nocturnal hypoglycemia was significantly lower with BIAsp70 vs BIAsp30 (1.23 vs 3.21 events/subject year; P = 0.0002) at week 28.
Conclusions: Thrice-daily BIAsp70 was superior to twice-daily BIAsp30 in terms of HbA1c change, with less variation in daytime plasma glucose profiles. BIAsp70 was well tolerated, with a lower incidence of nocturnal hypoglycemia vs BIAsp30. This trial was registered with ClinicalTrial.gov (no. NCT00318786). (J Diabetes Invest, doi: 10.1111/j.2040-1124.2010.00015.x, 2010).
Keywords: Biphasic insulin aspart 70; Postprandial hyperglycemia; Type 2 diabetes mellitus.
Figures
References
-
- Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986 - PubMed
-
- UK Prospective Diabetes Study Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853 - PubMed
-
- Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study. Diabetes Res Clin Pract 1995; 28: 103–117 - PubMed
-
- DECODE Study Group . Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. Lancet 1999; 354: 617–621 - PubMed
-
- Tominaga M, Eguchi H, Manaka H, et al. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care 1999; 22: 920–924 - PubMed
LinkOut - more resources
Full Text Sources
Medical