Synergistic effect of α-glucosidase inhibitors and dipeptidyl peptidase 4 inhibitor treatment
- PMID: 24843484
- PMCID: PMC4014919
- DOI: 10.1111/j.2040-1124.2010.00081.x
Synergistic effect of α-glucosidase inhibitors and dipeptidyl peptidase 4 inhibitor treatment
Abstract
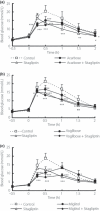
Monotherapy of α-glucosidase inhibitor (α-GI) or dipeptidyl peptidase 4 (DPP4) inhibitor does not sufficiently minimize glucose fluctuations in the diabetic state. In the present study, we evaluated the combined effects of various of α-GI inhibitors (acarbose, voglibose or miglitol) and sitagliptin, a DPP4 inhibitor, on blood glucose fluctuation, insulin and active glucagon-like peptide-1 (GLP-1) levels after nutriment loading in mice. Miglitol and sitagliptin elicited a 47% reduction (P < 0.05) of the area under the curve of blood glucose levels for up to 2 h after maltose-loading, a 60% reduction (P < 0.05) in the range of blood glucose fluctuation, and a 32% decrease in plasma insulin compared with the control group. All three of the combinations elicited a 2.5-4.9-fold synergistic increase in active GLP-1 (P < 0.05 vs control). Thus, combined treatment with the α-GI miglitol, which more strongly inhibits the early phase of postprandial hyperglycemia, and DPP4 inhibitor yields both complementary and synergistic effects, and might represent a superior anti-hyperglycemic therapy. (J Diabetes Invest, doi: 10.1111/j.2040-1124.2010.00081.x, 2011).
Keywords: Glucagon‐like peptide‐1; Insulin; Postprandial hyperglycemia.
Figures
References
-
- Chiasson JL, Josse RG, Gomis R, et al. Stop‐NIDDM Trail Research Group Acarbose prevention of type 2 diabetes mellitus: the STOP‐NIDDM randomised trial. Lancet 2002; 359: 2072–2077 - PubMed
-
- Narita T, Katsuura Y, Sato T, et al. Miglitol induces prolonged and enhanced glucagons‐like peptide‐1 and reduced gastric inhibitory polypeptide responses after ingestion of a mixed meal in Japanese Type 2 diabetic patients. Diabet Med 2009; 26: 187–188 - PubMed
-
- Esposito K, Ciotola M, Carleo D, et al. Post‐meal glucose peaks at home associate with carotid intima‐media thickness in type 2 diabetes. J Clin Endocrinol Metab 2008; 93: 1345–1350 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

