The once-daily human glucagon-like peptide-1 analog, liraglutide, improves β-cell function in Japanese patients with type 2 diabetes
- PMID: 24843595
- PMCID: PMC4019260
- DOI: 10.1111/j.2040-1124.2012.00193.x
The once-daily human glucagon-like peptide-1 analog, liraglutide, improves β-cell function in Japanese patients with type 2 diabetes
Abstract
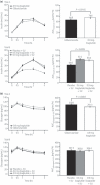
Aims/Introduction: β-cell function was evaluated by homeostasis model assessment of β-cell function (HOMA-B) index, proinsulin:insulin and proinsulin:C-peptide ratios in adult, Japanese type 2 diabetes patients receiving liraglutide.
Materials and methods: Data from two randomized, controlled clinical trials (A and B) including 664 Japanese type 2 diabetes patients (mean values: glycated hemoglobin [HbA1c] 8.61-9.32%; body mass index [BMI] 24.4-25.3 kg/m(2)) were analyzed. In two 24-week trials, patients received liraglutide 0.9 mg (n = 268) or glibenclamide 2.5 mg (n = 132; trial A), or liraglutide 0.6, 0.9 mg (n = 176) or placebo (n = 88) added to previous sulfonylurea therapy (trial B).
Results: Liraglutide was associated with improved glycemic control vs sulfonylurea monotherapy or placebo. In liraglutide-treated groups in trials A and B, area under the curve (AUC) insulin 0-3 h was improved (P < 0.001 for all) and the AUCinsulin 0-3 h:AUCglucose 0-3 h ratio was increased (estimated treatment difference [liraglutide-comparator] 0.058 [0.036, 0.079]). HOMA-B significantly increased with liraglutide relative to comparator in trial B (P < 0.05), but not in trial A. The reduction in fasting proinsulin:insulin ratio was 50% greater than in comparator groups.
Conclusions: In Japanese type 2 diabetes patients, liraglutide was associated with effective glycemic control, restoration of prandial insulin response and indications of improved β-cell function. This trial was registered with Clinicaltrials.gov (trial A: no. NCT00393718/JapicCTI-060328 and trial B: no. NCT00395746/JapicCTI-060324). (J Diabetes Invest, doi: 10.1111/j.2040-1124.2012.00193.x, 2012).
Keywords: Insulin‐secreting cells; Liraglutide; Type 2 diabetes.
Figures
References
-
- Morimoto A, Nishimura R, Tajima N. Trends in the epidemiology of patients with diabetes in Japan. Jpn Med Assoc J 2010; 53: 36–40
-
- Kawamori R. Diabetes trends in Japan. Diabetes Metab Res Rev 2002; 18(Suppl. 3): S9–S13 - PubMed
-
- Fukushima M, Usami M, Ikeda M, et al. Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross‐sectional study of Japanese type 2 diabetes. Metab Clin Exp 2004; 53: 831–835 - PubMed
-
- Kobayashi M, Yamazaki K, Hirao K, et al. The status of diabetes control and antidiabetic drug therapy in Japan – a cross‐sectional survey of 17,000 patients with diabetes mellitus (JDDM 1). Diabetes Res Clin Pract 2006; 73: 198–204 - PubMed
-
- Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin or glyburide monotherapy. N Engl J Med 2006; 355: 2427–2443 - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

