Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis
- PMID: 24844246
- PMCID: PMC4054777
- DOI: 10.7554/eLife.02523
Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis
Abstract
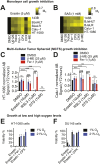
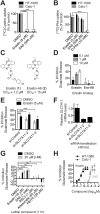
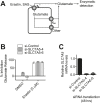
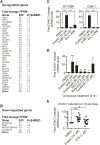
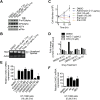
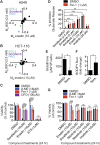
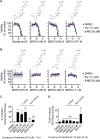
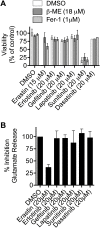
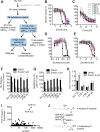
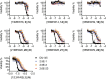
Exchange of extracellular cystine for intracellular glutamate by the antiporter system xc (-) is implicated in numerous pathologies. Pharmacological agents that inhibit system xc (-) activity with high potency have long been sought, but have remained elusive. In this study, we report that the small molecule erastin is a potent, selective inhibitor of system xc (-). RNA sequencing revealed that inhibition of cystine-glutamate exchange leads to activation of an ER stress response and upregulation of CHAC1, providing a pharmacodynamic marker for system xc (-) inhibition. We also found that the clinically approved anti-cancer drug sorafenib, but not other kinase inhibitors, inhibits system xc (-) function and can trigger ER stress and ferroptosis. In an analysis of hospital records and adverse event reports, we found that patients treated with sorafenib exhibited unique metabolic and phenotypic alterations compared to patients treated with other kinase-inhibiting drugs. Finally, using a genetic approach, we identified new genes dramatically upregulated in cells resistant to ferroptosis.DOI: http://dx.doi.org/10.7554/eLife.02523.001.
Keywords: SLC7A11; cell death; cystine; erastin; reactive oxygen species; sorafenib.
Copyright © 2014, Dixon et al.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures



References
-
- Agyeman AS, Chaerkady R, Shaw PG, Davidson NE, Visvanathan K, Pandey A, Kensler TW. 2012. Transcriptomic and proteomic profiling of KEAP1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast Cancer Research and Treatment 132:175–187. doi: 10.1007/s10549-011-1536-9 - DOI - PMC - PubMed
-
- Awada A, Hendlisz A, Gil T, Bartholomeus S, Mano M, de Valeriola D, Strumberg D, Brendel E, Haase CG, Schwartz B, Piccart M. 2005. Phase I safety and pharmacokinetics of BAY 43-9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. British Journal of Cancer 92:1855–1861. doi: 10.1038/sj.bjc.6602584 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

