Δ9-tetrahydrocannabinol prevents methamphetamine-induced neurotoxicity
- PMID: 24844285
- PMCID: PMC4028295
- DOI: 10.1371/journal.pone.0098079
Δ9-tetrahydrocannabinol prevents methamphetamine-induced neurotoxicity
Abstract
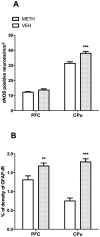
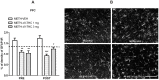
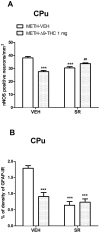
Methamphetamine (METH) is a potent psychostimulant with neurotoxic properties. Heavy use increases the activation of neuronal nitric oxide synthase (nNOS), production of peroxynitrites, microglia stimulation, and induces hyperthermia and anorectic effects. Most METH recreational users also consume cannabis. Preclinical studies have shown that natural (Δ9-tetrahydrocannabinol, Δ9-THC) and synthetic cannabinoid CB1 and CB2 receptor agonists exert neuroprotective effects on different models of cerebral damage. Here, we investigated the neuroprotective effect of Δ9-THC on METH-induced neurotoxicity by examining its ability to reduce astrocyte activation and nNOS overexpression in selected brain areas. Rats exposed to a METH neurotoxic regimen (4 × 10 mg/kg, 2 hours apart) were pre- or post-treated with Δ9-THC (1 or 3 mg/kg) and sacrificed 3 days after the last METH administration. Semi-quantitative immunohistochemistry was performed using antibodies against nNOS and Glial Fibrillary Acidic Protein (GFAP). Results showed that, as compared to corresponding controls (i) METH-induced nNOS overexpression in the caudate-putamen (CPu) was significantly attenuated by pre- and post-treatment with both doses of Δ9-THC (-19% and -28% for 1 mg/kg pre- and post-treated animals; -25% and -21% for 3 mg/kg pre- and post-treated animals); (ii) METH-induced GFAP-immunoreactivity (IR) was significantly reduced in the CPu by post-treatment with 1 mg/kg Δ9-THC1 (-50%) and by pre-treatment with 3 mg/kg Δ9-THC (-53%); (iii) METH-induced GFAP-IR was significantly decreased in the prefrontal cortex (PFC) by pre- and post-treatment with both doses of Δ9-THC (-34% and -47% for 1 mg/kg pre- and post-treated animals; -37% and -29% for 3 mg/kg pre- and post-treated animals). The cannabinoid CB1 receptor antagonist SR141716A attenuated METH-induced nNOS overexpression in the CPu, but failed to counteract the Δ9-THC-mediated reduction of METH-induced GFAP-IR both in the PFC and CPu. Our results indicate that Δ9-THC reduces METH-induced brain damage via inhibition of nNOS expression and astrocyte activation through CB1-dependent and independent mechanisms, respectively.
Conflict of interest statement
Figures





Similar articles
-
Differential role of the nitric oxide pathway on delta(9)-THC-induced central nervous system effects in the mouse.Eur J Neurosci. 2001 Feb;13(3):561-8. doi: 10.1046/j.1460-9568.2001.01431.x. Eur J Neurosci. 2001. PMID: 11168564
-
Effects of chronic exposure to delta9-tetrahydrocannabinol on cannabinoid receptor binding and mRNA levels in several rat brain regions.Brain Res Mol Brain Res. 1997 Jun;46(1-2):100-8. doi: 10.1016/s0169-328x(96)00277-x. Brain Res Mol Brain Res. 1997. PMID: 9191083
-
Neurotoxic-related changes in tyrosine hydroxylase, microglia, myelin, and the blood-brain barrier in the caudate-putamen from acute methamphetamine exposure.Synapse. 2008 Mar;62(3):193-204. doi: 10.1002/syn.20478. Synapse. 2008. PMID: 18081184
-
Possible mechanism for the neuroprotective effects of L-carnitine on methamphetamine-evoked neurotoxicity.Ann N Y Acad Sci. 2003 May;993:197-207; discussion 287-8. doi: 10.1111/j.1749-6632.2003.tb07530.x. Ann N Y Acad Sci. 2003. PMID: 12853314 Review.
-
New perspectives in the studies on endocannabinoid and cannabis: abnormal behaviors associate with CB1 cannabinoid receptor and development of therapeutic application.J Pharmacol Sci. 2004 Dec;96(4):362-6. doi: 10.1254/jphs.fmj04003x2. Epub 2004 Dec 10. J Pharmacol Sci. 2004. PMID: 15599103 Review.
Cited by
-
Bath salts and polyconsumption: in search of drug-drug interactions.Psychopharmacology (Berl). 2019 Mar;236(3):1001-1014. doi: 10.1007/s00213-019-05213-3. Epub 2019 Mar 25. Psychopharmacology (Berl). 2019. PMID: 30911791 Review.
-
Pharmacological Treatments for Methamphetamine Use Disorder: Current Status and Future Targets.Subst Abuse Rehabil. 2024 Aug 30;15:125-161. doi: 10.2147/SAR.S431273. eCollection 2024. Subst Abuse Rehabil. 2024. PMID: 39228432 Free PMC article. Review.
-
Repeated exposure to JWH-018 induces adaptive changes in the mesolimbic and mesocortical dopaminergic pathways, glial cells alterations, and behavioural correlates.Br J Pharmacol. 2021 Sep;178(17):3476-3497. doi: 10.1111/bph.15494. Epub 2021 Jun 29. Br J Pharmacol. 2021. PMID: 33837969 Free PMC article.
-
Repeated Administration of 3,4-Methylenedioxymethamphetamine (MDMA) Elevates the Levels of Neuronal Nitric Oxide Synthase in the Nigrostriatal System: Possible Relevance to Neurotoxicity.Neurotox Res. 2018 Nov;34(4):763-768. doi: 10.1007/s12640-018-9892-4. Epub 2018 Apr 9. Neurotox Res. 2018. PMID: 29629511
-
HIV-1, methamphetamine and astrocytes at neuroinflammatory Crossroads.Front Microbiol. 2015 Oct 27;6:1143. doi: 10.3389/fmicb.2015.01143. eCollection 2015. Front Microbiol. 2015. PMID: 26579077 Free PMC article. Review.
References
-
- United Nation Office on Drugs and Crime (UNODC) (2013) World Drug Report 2013 (United Nations publication, Sales No. E.13.XI.6).
-
- LaVoie MJ, Card JP, Hastings TG (2004) Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol 187: 47–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

