Designing allosteric inhibitors of factor XIa. Lessons from the interactions of sulfated pentagalloylglucopyranosides
- PMID: 24844380
- PMCID: PMC4216218
- DOI: 10.1021/jm500311e
Designing allosteric inhibitors of factor XIa. Lessons from the interactions of sulfated pentagalloylglucopyranosides
Abstract
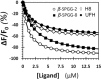
We recently introduced sulfated pentagalloylglucopyranoside (SPGG) as an allosteric inhibitor of factor XIa (FXIa) (Al-Horani et al., J. Med Chem. 2013, 56, 867-878). To better understand the SPGG-FXIa interaction, we utilized eight SPGG variants and a range of biochemical techniques. The results reveal that SPGG's sulfation level moderately affected FXIa inhibition potency and selectivity over thrombin and factor Xa. Variation in the anomeric configuration did not affect potency. Interestingly, zymogen factor XI bound SPGG with high affinity, suggesting its possible use as an antidote. Acrylamide quenching experiments suggested that SPGG induced significant conformational changes in the active site of FXIa. Inhibition studies in the presence of heparin showed marginal competition with highly sulfated SPGG variants but robust competition with less sulfated variants. Resolution of energetic contributions revealed that nonionic forces contribute nearly 87% of binding energy suggesting a strong possibility of specific interaction. Overall, the results indicate that SPGG may recognize more than one anion-binding, allosteric site on FXIa. An SPGG molecule containing approximately 10 sulfate groups on positions 2 through 6 of the pentagalloylglucopyranosyl scaffold may be the optimal FXIa inhibitor for further preclinical studies.
Figures









References
-
- Ruppert A.; Lees M.; Steinle T. Clinical burden of venous thromboembolism. Curr. Med. Res. Opin. 2010, 26, 2465–2473. - PubMed
-
- Nutescu E. A.; Dager W. E.; Kalus J. S.; Lewin J. J. III; Cipolle M. D. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am. J. Health Syst. Pharm. 2013, 70, 1914–1929. - PubMed
-
- Yates S.; Sarode R. Novel thrombin and factor Xa inhibitors: challenges to reversal of their anticoagulation effects. Curr. Opin. Hematol. 2013, 20, 552–557. - PubMed
-
- van Montfoort M. L.; Meijers J. C. M. Anticoagulation beyond direct thrombin and factor Xa inhibitors: indications for targeting the intrinsic pathway?. Thromb. Haemost. 2013, 110, 223–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

