Fungal cellulase is an elicitor but its enzymatic activity is not required for its elicitor activity
- PMID: 24844544
- PMCID: PMC6638370
- DOI: 10.1111/mpp.12156
Fungal cellulase is an elicitor but its enzymatic activity is not required for its elicitor activity
Abstract
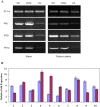
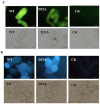
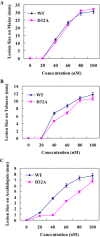
Plant-pathogenic fungi produce cellulases. However, little information is available on cellulase as an elicitor in plant-pathogen interactions. Here, an endocellulase (EG1) was isolated from Rhizoctonia solani. It contains a putative protein of 227 amino acids with a signal peptide and a family-45 glycosyl hydrolase domain. Its aspartic acid (Asp) residue at position 32 was changed to alanine (Ala), resulting in full loss of its catalytic activity. Wild-type and mutated forms of the endoglucanase were expressed in yeast and purified to homogeneity. The purified wild-type and mutant forms induced cell death in maize, tobacco and Arabidopsis leaves, and the transcription of three defence marker genes in maize and tobacco and 10 genes related to defence responses in maize. Moreover, they also induced the accumulation of reactive oxygen species (ROS), medium alkalinization, Ca(2+) accumulation and ethylene biosynthesis of suspension-cultured tobacco cells. Similarly, production of the EG1 wild-type and mutated forms in tobacco induced cell death using the Potato virus X (PVX) expression system. In vivo, expression of EG1 was also related to cell death during infection of maize by R. solani. These results provide direct evidence that the endoglucanase is an elicitor, but its enzymatic activity is not required for its elicitor activity.
Keywords: Rhizoctonia solani; elicitor; fungal cellulase.
© 2014 BSPP AND JOHN WILEY & SONS LTD.
Figures






Similar articles
-
Glycoside Hydrolase Family 16 Enzyme RsEG146 From Rhizoctonia solani AG1 IA Induces Cell Death and Triggers Defence Response in Nicotiana tabacum.Mol Plant Pathol. 2025 Mar;26(3):e70075. doi: 10.1111/mpp.70075. Mol Plant Pathol. 2025. PMID: 40091519 Free PMC article.
-
The enzymatic activity of fungal xylanase is not necessary for its elicitor activity.Plant Physiol. 1999 Oct;121(2):391-7. doi: 10.1104/pp.121.2.391. Plant Physiol. 1999. PMID: 10517830 Free PMC article.
-
A novel protein elicitor (SsCut) from Sclerotinia sclerotiorum induces multiple defense responses in plants.Plant Mol Biol. 2014 Nov;86(4-5):495-511. doi: 10.1007/s11103-014-0244-3. Epub 2014 Aug 23. Plant Mol Biol. 2014. PMID: 25149470
-
A novel elicitor identified from Magnaporthe oryzae triggers defense responses in tobacco and rice.Plant Cell Rep. 2014 Nov;33(11):1865-79. doi: 10.1007/s00299-014-1663-y. Epub 2014 Jul 24. Plant Cell Rep. 2014. PMID: 25056480
-
Suppression subtractive hybridization and comparative expression of a pore-forming toxin and glycosyl hydrolase genes in Rhizoctonia solani during potato sprout infection.Mol Genet Genomics. 2015 Jun;290(3):877-900. doi: 10.1007/s00438-014-0962-x. Epub 2014 Dec 4. Mol Genet Genomics. 2015. PMID: 25472038
Cited by
-
Fusarium oxysporum f. sp. niveum Pumilio 1 Regulates Virulence on Watermelon through Interacting with the ARP2/3 Complex and Binding to an A-Rich Motif in the 3' UTR of Diverse Transcripts.mBio. 2023 Apr 25;14(2):e0015723. doi: 10.1128/mbio.00157-23. Epub 2023 Mar 1. mBio. 2023. PMID: 36856417 Free PMC article.
-
An Endoglucanase Secreted by Ustilago esculenta Promotes Fungal Proliferation.J Fungi (Basel). 2022 Oct 7;8(10):1050. doi: 10.3390/jof8101050. J Fungi (Basel). 2022. PMID: 36294616 Free PMC article.
-
Unveiling the Core Effector Proteins of Oil Palm Pathogen Ganoderma boninense via Pan-Secretome Analysis.J Fungi (Basel). 2022 Jul 29;8(8):793. doi: 10.3390/jof8080793. J Fungi (Basel). 2022. PMID: 36012782 Free PMC article.
-
Genome-Wide Identification of M35 Family Metalloproteases in Rhizoctonia cerealis and Functional Analysis of RcMEP2 as a Virulence Factor during the Fungal Infection to Wheat.Int J Mol Sci. 2020 Apr 23;21(8):2984. doi: 10.3390/ijms21082984. Int J Mol Sci. 2020. PMID: 32340265 Free PMC article.
-
A mutation in an exoglucanase of Xanthomonas oryzae pv. oryzae, which confers an endo mode of activity, affects bacterial virulence, but not the induction of immune responses, in rice.Mol Plant Pathol. 2018 Jun;19(6):1364-1376. doi: 10.1111/mpp.12620. Epub 2017 Dec 26. Mol Plant Pathol. 2018. PMID: 28976110 Free PMC article.
References
-
- Ahuja, I. , Kissen, R. and Bones, A.M. (2012) Phytoalexins in defense against pathogens. Trends Plant Sci. 17, 73–90. - PubMed
-
- Angelova, Z. , Georgiev, S. and Roos, W. (2006) Elicitation of plants. Biotechnol. Biotechnol. Equip. 20, 72–83.
-
- Annis, S.L. and Goodwin, P.H. (1997) Recent advances in the molecular genetics of plant cell wall‐degrading enzymes produced by plant pathogenic fungi. Eur. J. Plant Pathol. 103, 1–14.
-
- Ausubel, F.M. (2005) Are innate immune signaling pathways in plants and animals conserved? Nat. Immunol. 6, 973–979. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

