Etanercept blocks inflammatory responses orchestrated by TNF-α to promote transplanted cell engraftment and proliferation in rat liver
- PMID: 24844924
- PMCID: PMC4176524
- DOI: 10.1002/hep.27232
Etanercept blocks inflammatory responses orchestrated by TNF-α to promote transplanted cell engraftment and proliferation in rat liver
Abstract
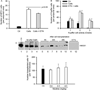
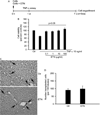
Engraftment of transplanted cells is critical for liver-directed cell therapy, but most transplanted cells are rapidly cleared from liver sinusoids by proinflammatory cytokines/chemokines/receptors after activation of neutrophils or Kupffer cells (KCs). To define whether tumor necrosis factor alpha (TNF-α) served roles in cell-transplantation-induced hepatic inflammation, we used the TNF-α antagonist, etanercept (ETN), for studies in syngeneic rat hepatocyte transplantation systems. After cell transplantation, multiple cytokines/chemokines/receptors were overexpressed, whereas ETN before cell transplantation essentially normalized these responses. Moreover, ETN down-regulated cell-transplantation-induced intrahepatic release of secretory cytokines, such as high-mobility group box 1. These effects of ETN decreased cell-transplantation-induced activation of neutrophils, but not of KCs. Transplanted cell engraftment improved by several-fold in ETN-treated animals. These gains in cell engraftment were repeatedly realized after pretreatment of animals with ETN before multiple cell transplantation sessions. Transplanted cell numbers did not change over time, indicating absence of cell proliferation after ETN alone. By contrast, in animals preconditioned with retrorsine and partial hepatectomy, cell transplantation after ETN pretreatment significantly accelerated liver repopulation, compared to control rats.
Conclusion: TNF-α plays a major role in orchestrating cell-transplantation-induced inflammation through regulation of multiple cytokines/chemokines/receptor expression. Because TNF-α antagonism by ETN decreased transplanted cell clearance, improved cell engraftment, and accelerated liver repopulation, this pharmacological approach to control hepatic inflammation will help optimize clinical strategies for liver cell therapy.
© 2014 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Figures




Similar articles
-
Endothelin-1 receptor A blocker darusentan decreases hepatic changes and improves liver repopulation after cell transplantation in rats.Hepatology. 2014 Mar;59(3):1107-17. doi: 10.1002/hep.26766. Epub 2014 Jan 16. Hepatology. 2014. PMID: 24114775 Free PMC article.
-
Hepatocyte transplantation-induced liver inflammation is driven by cytokines-chemokines associated with neutrophils and Kupffer cells.Gastroenterology. 2009 May;136(5):1806-17. doi: 10.1053/j.gastro.2009.01.063. Gastroenterology. 2009. PMID: 19422086 Free PMC article.
-
Kupffer cells participate in early clearance of syngeneic hepatocytes transplanted in the rat liver.Gastroenterology. 2002 Nov;123(5):1677-85. doi: 10.1053/gast.2002.36592. Gastroenterology. 2002. PMID: 12404242
-
Mechanisms of action of etanercept in psoriasis.J Investig Dermatol Symp Proc. 2007 May;12(1):38-45. doi: 10.1038/sj.jidsymp.5650037. J Investig Dermatol Symp Proc. 2007. PMID: 17502868 Review.
-
Etanercept, a novel drug for the treatment of patients with severe, active rheumatoid arthritis.Clin Ther. 1999 Jan;21(1):75-87; discussion 1-2. doi: 10.1016/S0149-2918(00)88269-7. Clin Ther. 1999. PMID: 10090426 Review.
Cited by
-
Cell therapy in chronic liver disease.Curr Opin Gastroenterol. 2016 May;32(3):189-94. doi: 10.1097/MOG.0000000000000262. Curr Opin Gastroenterol. 2016. PMID: 26950359 Free PMC article. Review.
-
Angelica sinensis Polysaccharide and Astragalus membranaceus Polysaccharide Accelerate Liver Regeneration by Enhanced Glycolysis via Activation of JAK2/STAT3/HK2 Pathway.Molecules. 2022 Nov 15;27(22):7890. doi: 10.3390/molecules27227890. Molecules. 2022. PMID: 36431990 Free PMC article.
-
Diagnostic and therapeutic dilemma in Stevens-Johnson syndrome-like acute graft-versus-host disease after liver transplantation: A case report.Front Immunol. 2022 Aug 18;13:917782. doi: 10.3389/fimmu.2022.917782. eCollection 2022. Front Immunol. 2022. PMID: 36059444 Free PMC article.
-
Thalidomide promotes transplanted cell engraftment in the rat liver by modulating inflammation and endothelial integrity.J Hepatol. 2016 Dec;65(6):1171-1178. doi: 10.1016/j.jhep.2016.07.008. Epub 2016 Jul 12. J Hepatol. 2016. PMID: 27422749 Free PMC article.
-
Kupffer Cell Transplantation in Mice for Elucidating Monocyte/Macrophage Biology and for Potential in Cell or Gene Therapy.Am J Pathol. 2016 Mar;186(3):539-51. doi: 10.1016/j.ajpath.2015.11.002. Epub 2016 Jan 7. Am J Pathol. 2016. PMID: 26773351 Free PMC article.
References
-
- Filippi C, Dhawan A. Current status of human hepatocyte transplantation and its potential for Wilson's disease. Ann N Y Acad Sci. 2014 Mar 7; [Epub ahead of print] - PubMed
-
- Rajvanshi P, Kerr A, Bhargava KK, Burk RD, Gupta S. Efficacy and safety of repeated hepatocyte transplantation for significant liver repopulation in rodents. Gastroenterology. 1996;111:1092–1102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
