Probing structure-function relationships in missense variants in the carboxy-terminal region of BRCA1
- PMID: 24845084
- PMCID: PMC4028255
- DOI: 10.1371/journal.pone.0097766
Probing structure-function relationships in missense variants in the carboxy-terminal region of BRCA1
Abstract
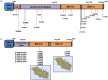
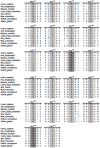
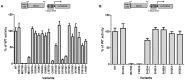
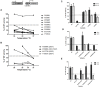
Germline inactivating variants in BRCA1 lead to a significantly increased risk of breast and ovarian cancers in carriers. While the functional effect of many variants can be inferred from the DNA sequence, determining the effect of missense variants present a significant challenge. A series of biochemical and cell biological assays have been successfully used to explore the impact of these variants on the function of BRCA1, which contribute to assessing their likelihood of pathogenicity. It has been determined that variants that co-localize with structural or functional motifs are more likely to disrupt the stability and function of BRCA1. Here we assess the functional impact of 37 variants chosen to probe the functional impact of variants in phosphorylation sites and in the BRCT domains. In addition, we perform a meta-analysis of 170 unique variants tested by the transcription activation assays in the carboxy-terminal domain of BRCA1 using a recently developed computation model to provide assessment for functional impact and their likelihood of pathogenicity.
Conflict of interest statement
Figures
References
-
- Frank TS, Deffenbaugh AM, Reid JE, Hulick M, Ward BE, et al. (2002) Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. Journal of Clinical Oncology 20: 1480–1490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

