Development and progress of Ireland's biobank network: Ethical, legal, and social implications (ELSI), standardized documentation, sample and data release, and international perspective
- PMID: 24845249
- PMCID: PMC4076973
- DOI: 10.1089/bio.2012.0028
Development and progress of Ireland's biobank network: Ethical, legal, and social implications (ELSI), standardized documentation, sample and data release, and international perspective
Abstract
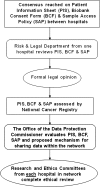
Biobank Ireland Trust (BIT) was established in 2004 to promote and develop an Irish biobank network to benefit patients, researchers, industry, and the economy. The network commenced in 2008 with two hospital biobanks and currently consists of biobanks in the four main cancer hospitals in Ireland. The St. James's Hospital (SJH) Biobank coordinates the network. Procedures, based on ISBER and NCI guidelines, are standardized across the network. Policies and documents-Patient Consent Policy, Patient Information Sheet, Biobank Consent Form, Sample and Data Access Policy (SAP), and Sample Application Form have been agreed upon (after robust discussion) for use in each hospital. An optimum sequence for document preparation and submission for review is outlined. Once consensus is reached among the participating biobanks, the SJH biobank liaises with the Research and Ethics Committees, the Office of the Data Protection Commissioner, The National Cancer Registry (NCR), patient advocate groups, researchers, and other stakeholders. The NCR provides de-identified data from its database for researchers via unique biobank codes. ELSI issues discussed include the introduction of prospective consent across the network and the return of significant research results to patients. Only 4 of 363 patients opted to be re-contacted and re-consented on each occasion that their samples are included in a new project. It was decided, after multidisciplinary discussion, that results will not be returned to patients. The SAP is modeled on those of several international networks. Biobank Ireland is affiliated with international biobanking groups-Marble Arch International Working Group, ISBER, and ESBB. The Irish government continues to deliberate on how to fund and implement biobanking nationally. Meanwhile BIT uses every opportunity to promote awareness of the benefits of biobanking in events and in the media.
Similar articles
-
"It's all about trust": reflections of researchers on the complexity and controversy surrounding biobanking in South Africa.BMC Med Ethics. 2016 Oct 10;17(1):57. doi: 10.1186/s12910-016-0140-2. BMC Med Ethics. 2016. PMID: 27724893 Free PMC article.
-
Determining the state of guidance on pediatric biobanking for researchers, HRECS, and families: Regulatory mapping of international guidance.Eur J Pediatr. 2024 May;183(5):2477-2490. doi: 10.1007/s00431-024-05469-8. Epub 2024 Mar 13. Eur J Pediatr. 2024. PMID: 38478133 Free PMC article. Review.
-
Establishment of a network-based intra-hospital virtual cancer biobank.Biopreserv Biobank. 2015 Feb;13(1):43-8. doi: 10.1089/bio.2014.0086. Biopreserv Biobank. 2015. PMID: 25686047
-
Stakeholder perspectives on the ethico-legal dimensions of biobanking in South Africa.BMC Med Ethics. 2021 Jul 1;22(1):84. doi: 10.1186/s12910-021-00645-z. BMC Med Ethics. 2021. PMID: 34210291 Free PMC article.
-
Balancing the local and the universal in maintaining ethical access to a genomics biobank.BMC Med Ethics. 2017 Dec 28;18(1):80. doi: 10.1186/s12910-017-0240-7. BMC Med Ethics. 2017. PMID: 29282045 Free PMC article. Review.
Cited by
-
Ethical implications of using biobanks and population databases for genetic suicide research.Am J Med Genet B Neuropsychiatr Genet. 2019 Dec;180(8):601-608. doi: 10.1002/ajmg.b.32718. Epub 2019 Feb 18. Am J Med Genet B Neuropsychiatr Genet. 2019. PMID: 30779308 Free PMC article. Review.
-
Returning Results: Let's Be Honest!Genet Test Mol Biomarkers. 2017 Mar;21(3):134-139. doi: 10.1089/gtmb.2016.0395. Epub 2017 Feb 24. Genet Test Mol Biomarkers. 2017. PMID: 28306398 Free PMC article.
-
EPMA position paper in cancer: current overview and future perspectives.EPMA J. 2015 Apr 15;6(1):9. doi: 10.1186/s13167-015-0030-6. eCollection 2015. EPMA J. 2015. PMID: 25908947 Free PMC article.
-
Assessing Researcher Needs for a Virtual Biobank.Biopreserv Biobank. 2017 Jun;15(3):203-210. doi: 10.1089/bio.2016.0009. Epub 2016 Dec 8. Biopreserv Biobank. 2017. PMID: 27929677 Free PMC article.
-
What GDPR and the Health Research Regulations (HRRs) mean for Ireland: a research perspective.Ir J Med Sci. 2021 May;190(2):505-514. doi: 10.1007/s11845-020-02330-3. Epub 2020 Jul 29. Ir J Med Sci. 2021. PMID: 32728834 Free PMC article.
References
-
- Department of Children and Health. A Strategy for Cancer Control in Ireland. National Cancer Form. 2006. http://www.hse.ie/eng/services/Publications/HealthProtection/Public_Heal.... [Sep 20;2012 ]. http://www.hse.ie/eng/services/Publications/HealthProtection/Public_Heal...
-
- Department of Children and Health. Recommendations for the Establishment of a National Cancer Biobank—Report of the Expert Group on a National Cancer Biobank. 2008. http://www.hrb.ie/uploads/tx_hrbpublications/Recommendations_for_the_Est.... http://www.hrb.ie/uploads/tx_hrbpublications/Recommendations_for_the_Est...
-
- Organization for Economic Co-operation and Development. OECD Guidelines on Human Biobanks and Genetic Research Databases. 2009. - PubMed
-
- Organization for Economic Co-operation and Development. OECD Best Practice Guidelines for Biological Resource Centers. 2007.
-
- Best Practices for Repositories—Collection, Storage, Retrieval and Distribution of Biological Materials for Research. Cell Preserv Technol. 2008;6:3–58.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous