Evaluation of conoscopic holography for estimating tumor resection cavities in model-based image-guided neurosurgery
- PMID: 24845293
- PMCID: PMC4185972
- DOI: 10.1109/TBME.2014.2308299
Evaluation of conoscopic holography for estimating tumor resection cavities in model-based image-guided neurosurgery
Abstract
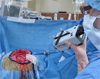
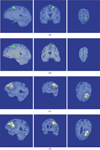
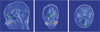
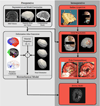
Surgical navigation relies on accurately mapping the intraoperative state of the patient to models derived from preoperative images. In image-guided neurosurgery, soft tissue deformations are common and have been shown to compromise the accuracy of guidance systems. In lieu of whole-brain intraoperative imaging, some advocate the use of intraoperatively acquired sparse data from laser-range scans, ultrasound imaging, or stereo reconstruction coupled with a computational model to drive subsurface deformations. Some authors have reported on compensating for brain sag, swelling, retraction, and the application of pharmaceuticals such as mannitol with these models. To date, strategies for modeling tissue resection have been limited. In this paper, we report our experiences with a novel digitization approach, called a conoprobe, to document tissue resection cavities and assess the impact of resection on model-based guidance systems. Specifically, the conoprobe was used to digitize the interior of the resection cavity during eight brain tumor resection surgeries and then compared against model prediction results of tumor locations. We should note that no effort was made to incorporate resection into the model but rather the objective was to determine if measurement was possible to study the impact on modeling tissue resection. In addition, the digitized resection cavity was compared with early postoperative MRI scans to determine whether these scans can further inform tissue resection. The results demonstrate benefit in model correction despite not having resection explicitly modeled. However, results also indicate the challenge that resection provides for model-correction approaches. With respect to the digitization technology, it is clear that the conoprobe provides important real-time data regarding resection and adds another dimension to our noncontact instrumentation framework for soft-tissue deformation compensation in guidance systems.
Figures



Similar articles
-
Clinical evaluation of a model-updated image-guidance approach to brain shift compensation: experience in 16 cases.Int J Comput Assist Radiol Surg. 2016 Aug;11(8):1467-74. doi: 10.1007/s11548-015-1295-x. Epub 2015 Oct 17. Int J Comput Assist Radiol Surg. 2016. PMID: 26476637 Free PMC article.
-
Persistent and automatic intraoperative 3D digitization of surfaces under dynamic magnifications of an operating microscope.Med Image Anal. 2015 Jan;19(1):30-45. doi: 10.1016/j.media.2014.07.004. Epub 2014 Aug 7. Med Image Anal. 2015. PMID: 25189364 Free PMC article.
-
Comparison study of intraoperative surface acquisition methods for surgical navigation.IEEE Trans Biomed Eng. 2013 Apr;60(4):1090-9. doi: 10.1109/TBME.2012.2215033. Epub 2012 Aug 23. IEEE Trans Biomed Eng. 2013. PMID: 22929367 Free PMC article.
-
IBIS: an OR ready open-source platform for image-guided neurosurgery.Int J Comput Assist Radiol Surg. 2017 Mar;12(3):363-378. doi: 10.1007/s11548-016-1478-0. Epub 2016 Aug 31. Int J Comput Assist Radiol Surg. 2017. PMID: 27581336 Review.
-
Optimizing brain tumor resection. Midfield interventional MR imaging.Neuroimaging Clin N Am. 2001 Nov;11(4):659-72. Neuroimaging Clin N Am. 2001. PMID: 11995421 Review.
Cited by
-
Patient Registration Using Intraoperative Stereovision in Image-guided Open Spinal Surgery.IEEE Trans Biomed Eng. 2015 Sep;62(9):2177-86. doi: 10.1109/TBME.2015.2415731. Epub 2015 Mar 26. IEEE Trans Biomed Eng. 2015. PMID: 25826802 Free PMC article.
-
Clinical evaluation of a model-updated image-guidance approach to brain shift compensation: experience in 16 cases.Int J Comput Assist Radiol Surg. 2016 Aug;11(8):1467-74. doi: 10.1007/s11548-015-1295-x. Epub 2015 Oct 17. Int J Comput Assist Radiol Surg. 2016. PMID: 26476637 Free PMC article.
-
Surface scanning for 3D dose calculation in intraoperative electron radiation therapy.Radiat Oncol. 2018 Dec 7;13(1):243. doi: 10.1186/s13014-018-1181-0. Radiat Oncol. 2018. PMID: 30526626 Free PMC article.
-
Accurate three-dimensional virtual reconstruction of surgical field using calibrated trajectories of an image-guided medical robot.J Med Imaging (Bellingham). 2014 Oct;1(3):035002. doi: 10.1117/1.JMI.1.3.035002. Epub 2014 Dec 2. J Med Imaging (Bellingham). 2014. PMID: 26158071 Free PMC article.
-
ConoSurf: Open-source 3D scanning system based on a conoscopic holography device for acquiring surgical surfaces.Int J Med Robot. 2017 Sep;13(3):e1788. doi: 10.1002/rcs.1788. Epub 2016 Nov 21. Int J Med Robot. 2017. PMID: 27868345 Free PMC article.
References
-
- Bucholz RD, Yeh DD, Trobaugh J, McDurmont LL, Sturm CD, Baumann C, Henderson JM, Levy A, Kessma P. The Correction of Stereotactic Inaccuracy Caused by Brain Shift Using an Intraoperative Ultrasound Device; Proc. First Joint Conf. Comput. Vision, Virtual Reality and Robotics in Medicine and Medical Robotics and Comput.-Assisted Surgery; 1997. pp. 459–466.
-
- Burgner J, Simpson AL, Fitzpatrick JM, Lathrop RA, Herrell SD, Miga MI, Webster RJ., III A study on the theoretical and practical accuracy of conoscopic holography-based surface measurements: Toward image registration in minimally invasive surgery. Int. J. Med. Robot. Comput.-Assisted Surg. 2013;9:190–203. - PMC - PubMed
-
- Cash DM, Miga MI, Sinha TK, Galloway RL, Chapman WC. Compensating for intra-operative soft tissue deformations using incomplete surface data and finite elements. IEEE Trans. Med. Imag. 2005 Nov.24(11):1479–1491. - PubMed
-
- Collignon A, Maes F, Delaere D, Vandermeulen D, Suetens P, Marchal G. Information Processing in Medical Imaging. Dordrecht, The Netherlands: Kluwer; 1995. Automated multimodality image registration based on information theory; pp. 263–274.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

