Fungal polyketide synthase product chain-length control by partnering thiohydrolase
- PMID: 24845309
- PMCID: PMC4215887
- DOI: 10.1021/cb500284t
Fungal polyketide synthase product chain-length control by partnering thiohydrolase
Abstract
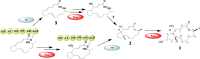
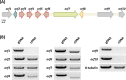
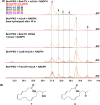
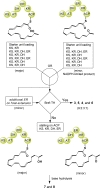
Fungal highly reducing polyketide synthases (HRPKSs) are an enigmatic group of multidomain enzymes that catalyze the biosynthesis of structurally diverse compounds. This variety stems from their intrinsic programming rules, which permutate the use of tailoring domains and determine the overall number of iterative cycles. From genome sequencing and mining of the producing strain Eupenicillium brefeldianum ATCC 58665, we identified an HRPKS involved in the biosynthesis of an important protein transport-inhibitor Brefeldin A (BFA), followed by reconstitution of its activity in Saccharomyces cerevisiae and in vitro. Bref-PKS demonstrated an NADPH-dependent reductive tailoring specificity that led to the synthesis of four different octaketide products with varying degrees of reduction. Furthermore, contrary to what is expected from the structure of BFA, Bref-PKS is found to be a nonaketide synthase in the absence of an associated thiohydrolase Bref-TH. Such chain-length control by the partner thiohydrolase was found to be present in other HRPKS systems and highlights the importance of including tailoring enzyme activities in predicting fungal HRPKS functions and their products.
Figures


Similar articles
-
Intrinsic and Extrinsic Programming of Product Chain Length and Release Mode in Fungal Collaborating Iterative Polyketide Synthases.J Am Chem Soc. 2020 Oct 7;142(40):17093-17104. doi: 10.1021/jacs.0c07050. Epub 2020 Sep 23. J Am Chem Soc. 2020. PMID: 32833442 Free PMC article.
-
Analysis of intact and dissected fungal polyketide synthase-nonribosomal peptide synthetase in vitro and in Saccharomyces cerevisiae.J Am Chem Soc. 2010 Oct 6;132(39):13604-7. doi: 10.1021/ja107084d. J Am Chem Soc. 2010. PMID: 20828130 Free PMC article.
-
Fungal Highly Reducing Polyketide Synthases Biosynthesize Salicylaldehydes That Are Precursors to Epoxycyclohexenol Natural Products.J Am Chem Soc. 2019 Dec 18;141(50):19538-19541. doi: 10.1021/jacs.9b09669. Epub 2019 Dec 5. J Am Chem Soc. 2019. PMID: 31790246 Free PMC article.
-
Yeast-based genome mining, production and mechanistic studies of the biosynthesis of fungal polyketide and peptide natural products.Nat Prod Rep. 2013 Aug;30(8):1139-49. doi: 10.1039/c3np70037b. Epub 2013 Jul 2. Nat Prod Rep. 2013. PMID: 23824111 Review.
-
Advances in cloning, functional analysis and heterologous expression of fungal polyketide synthase genes.J Biotechnol. 2006 Aug 5;124(4):690-703. doi: 10.1016/j.jbiotec.2006.03.046. Epub 2006 May 22. J Biotechnol. 2006. PMID: 16716432 Review.
Cited by
-
Phytotoxic Metabolites Produced by Legume-Associated Ascochyta and Its Related Genera in the Dothideomycetes.Toxins (Basel). 2019 Oct 29;11(11):627. doi: 10.3390/toxins11110627. Toxins (Basel). 2019. PMID: 31671808 Free PMC article. Review.
-
Biosynthesis of ilamycins featuring unusual building blocks and engineered production of enhanced anti-tuberculosis agents.Nat Commun. 2017 Aug 30;8(1):391. doi: 10.1038/s41467-017-00419-5. Nat Commun. 2017. PMID: 28855504 Free PMC article.
-
Synthetic biology of fungal natural products.Front Microbiol. 2015 Jul 30;6:775. doi: 10.3389/fmicb.2015.00775. eCollection 2015. Front Microbiol. 2015. PMID: 26284053 Free PMC article. Review.
-
Biochemical dissection of a fungal highly reducing polyketide synthase condensing region reveals basis for acyl group selection.Chem Sci. 2025 Jun 23;16(29):13173-13182. doi: 10.1039/d5sc01027f. eCollection 2025 Jul 23. Chem Sci. 2025. PMID: 40584239 Free PMC article.
-
Genomic Analysis of the Xanthoria elegans and Polyketide Synthase Gene Mining Based on the Whole Genome.Mycobiology. 2023 Feb 15;51(1):36-48. doi: 10.1080/12298093.2023.2175428. eCollection 2023. Mycobiology. 2023. PMID: 36846628 Free PMC article.
References
-
- Marinelli F. (2009) From Microbial Products to Novel Drugs that Target a Multitude of Disease Indications, in Methods in Enzymology (David A. H., Ed.), Chapter 2, pp 29–58, Academic Press, New York. - PubMed
-
- Cox R. J. (2007) Polyketides, proteins and genes in fungi: programmed nano-machines begin to reveal their secrets. Org. Biomol. Chem. 5, 2010–2026. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

