Members of the thrombospondin gene family bind stromal interaction molecule 1 and regulate calcium channel activity
- PMID: 24845346
- PMCID: PMC4502920
- DOI: 10.1016/j.matbio.2014.05.004
Members of the thrombospondin gene family bind stromal interaction molecule 1 and regulate calcium channel activity
Abstract
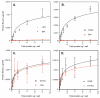
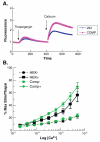
The thrombospondins (TSPs) are a family of matricellular proteins that regulate cellular phenotype through interactions with a myriad of other proteins and proteoglycans. We have identified a novel interaction of the members of the TSP gene family with stromal interaction molecule 1 (STIM1). This association is robust since it is preserved in Triton X-100, can be detected with multiple anti-TSP-1 and anti-STIM1 antibodies, and is detected in a wide range of cell types. We have also found that STIM1 co-immunoprecipitates with TSP-4 and cartilage oligomeric matrix protein (COMP), and that a recombinant version of the N-terminal domain of STIM1 binds to the signature domain of TSP-1 and COMP. The association of the TSPs with STIM1 is observed in both the presence and absence of calcium indicating that the calcium-dependent conformation of the signature domain of TSPs is not required for binding. Thus, this interaction could occur in the ER under conditions of normal or low calcium concentration. Furthermore, we observed that the expression of COMP in HEK 293 cells decreases STIM1-mediated calcium release activated calcium (CRAC) channel currents and increases arachidonic acid calcium (ARC) channel currents. These data indicate that the TSPs regulate STIM1 function and participate in the reciprocal regulation of two channels that mediate calcium entry into the cell.
Keywords: Arachidonate-regulated calcium channel; Calcium signaling; Stromal interaction molecule 1; Thrombospondin.
Copyright © 2014 The Authors. Published by Elsevier B.V. All rights reserved.
Figures





References
-
- Bale MD, Mosher DF. Thrombospondin is a substrate for blood coagulation factor XIIIa. Biochemistry. 1986;25:5667–5673. - PubMed
-
- Bornstein P, O’Rourke K, Wilstrom L, Wolf FW, Katz R, Li P, Dixit VM. A second, expressed thrombospondin gene (Thbs2) exists in the mouse genome. J. Biol. Chem. 1991;266:12821–12824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

