Systemic lipopolysaccharide induces cochlear inflammation and exacerbates the synergistic ototoxicity of kanamycin and furosemide
- PMID: 24845404
- PMCID: PMC4141430
- DOI: 10.1007/s10162-014-0458-8
Systemic lipopolysaccharide induces cochlear inflammation and exacerbates the synergistic ototoxicity of kanamycin and furosemide
Abstract
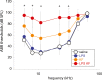
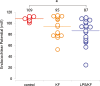
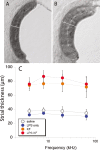
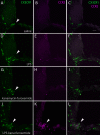
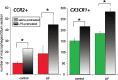
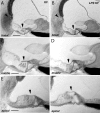
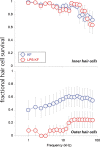
Aminoglycoside antibiotics are highly effective agents against gram-negative bacterial infections, but they cause adverse effects on hearing and balance dysfunction as a result of toxicity to hair cells of the cochlea and vestibular organs. While ototoxicity has been comprehensively studied, the contributions of the immune system, which controls the host response to infection, have not been studied in antibiotic ototoxicity. Recently, it has been shown that an inflammatory response is induced by hair cell injury. In this study, we found that lipopolysaccharide (LPS), an important component of bacterial endotoxin, when given in combination with kanamycin and furosemide, augmented the inflammatory response to hair cell injury and exacerbated hearing loss and hair cell injury. LPS injected into the peritoneum of experimental mice induced a brisk cochlear inflammatory response with recruitment of mononuclear phagocytes into the spiral ligament, even in the absence of ototoxic agents. While LPS alone did not affect hearing, animals that received LPS prior to ototoxic agents had worse hearing loss compared to those that did not receive LPS pretreatment. The poorer hearing outcome in LPS-treated mice did not correlate to changes in endocochlear potential. However, LPS-treated mice demonstrated an increased number of CCR2(+) inflammatory monocytes in the inner ear when compared with mice treated with ototoxic agents alone. We conclude that LPS and its associated inflammatory response are harmful to the inner ear when coupled with ototoxic medications and that the immune system may contribute to the final hearing outcome in subjects treated with ototoxic agents.
Figures

Similar articles
-
Comparative analysis of combination kanamycin-furosemide versus kanamycin alone in the mouse cochlea.Hear Res. 2011 Feb;272(1-2):108-16. doi: 10.1016/j.heares.2010.10.011. Epub 2010 Oct 31. Hear Res. 2011. PMID: 21044672 Free PMC article.
-
Expression of fractalkine receptor CX3CR1 on cochlear macrophages influences survival of hair cells following ototoxic injury.J Assoc Res Otolaryngol. 2010 Jun;11(2):223-34. doi: 10.1007/s10162-009-0198-3. Epub 2009 Nov 21. J Assoc Res Otolaryngol. 2010. PMID: 19936834 Free PMC article.
-
Kanamycin-furosemide ototoxicity in the mouse cochlea: a 3-dimensional analysis.Otolaryngol Head Neck Surg. 2014 Apr;150(4):666-72. doi: 10.1177/0194599813519071. Epub 2014 Jan 10. Otolaryngol Head Neck Surg. 2014. PMID: 24415490
-
Ototoxicity in developing mammals.Brain Res Brain Res Rev. 1995 Jan;20(1):68-90. doi: 10.1016/0165-0173(94)00006-b. Brain Res Brain Res Rev. 1995. PMID: 7711768 Review.
-
Ototoxicity: a high risk to auditory function that needs to be monitored in drug development.Front Mol Neurosci. 2024 May 2;17:1379743. doi: 10.3389/fnmol.2024.1379743. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38756707 Free PMC article. Review.
Cited by
-
A Novel Mouse Model of Aminoglycoside-Induced Hyperacusis and Tinnitus.Front Neurosci. 2020 Sep 18;14:561185. doi: 10.3389/fnins.2020.561185. eCollection 2020. Front Neurosci. 2020. PMID: 33041759 Free PMC article.
-
Adverse outcome pathway for aminoglycoside ototoxicity in drug-resistant tuberculosis treatment.Arch Toxicol. 2019 May;93(5):1385-1399. doi: 10.1007/s00204-019-02407-8. Epub 2019 Apr 8. Arch Toxicol. 2019. PMID: 30963202 Free PMC article.
-
Innate Immunity to Spiral Ganglion Neuron Loss: A Neuroprotective Role of Fractalkine Signaling in Injured Cochlea.Front Cell Neurosci. 2021 Aug 2;15:694292. doi: 10.3389/fncel.2021.694292. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34408629 Free PMC article. Review.
-
The cumulative effects of intravenous antibiotic treatments on hearing in patients with cystic fibrosis.J Cyst Fibros. 2017 May;16(3):401-409. doi: 10.1016/j.jcf.2017.01.006. Epub 2017 Feb 24. J Cyst Fibros. 2017. PMID: 28238634 Free PMC article.
-
Consumption of betel quid contributes to sensorineural hearing impairment through arecoline-induced oxidative stress.Sci Rep. 2019 Oct 10;9(1):14554. doi: 10.1038/s41598-019-49815-5. Sci Rep. 2019. PMID: 31601870 Free PMC article.
References
-
- Asakuma S, Snow JB., Jr Effects of furosemide and ethacrynic acid on the endocochlear direct current potential in normal and kanamycin sulfate-treated guinea pigs. Otolaryngol Head Neck Surg. 1980;88(2):188–193. - PubMed
-
- Bauerfeld CP, Rastogi R, Pirockinaite G, Lee I, Huttemann M, Monks B, Birnbaum MJ, Franchi L, Nunez G, Samavati L. TLR4-mediated AKT activation is MyD88/TRIF dependent and critical for induction of oxidative phosphorylation and mitochondrial transcription factor A in murine macrophages. J Immunol. 2012;188(6):2847–2857. doi: 10.4049/jimmunol.1102157. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

