Stress sensitivity and mechanotransduction during heart development
- PMID: 24845682
- PMCID: PMC4411556
- DOI: 10.1016/j.cub.2014.04.027
Stress sensitivity and mechanotransduction during heart development
Abstract
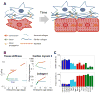
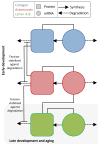
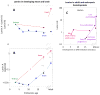
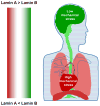
Early in embryogenesis, the heart begins its rhythmic contractions as a tube that helps perfuse the nascent vasculature, but the embryonic heart soon changes shape and mechanical properties, like many other developing organs. A key question in the field is whether stresses in development impact the underlying gene circuits and, if so, how? Here, we attempt to address this question as we review the mechanical maturation of heart - and, to a limited extent, lung and blood - with a focus on a few key abundant structural proteins whose expression dynamics have been suggested to be directly sensitive to mechanical stress. In heart maturation, proliferating fibroblasts deposit increasing amounts of collagenous matrix in parallel with cardiomyocytes expressing more sarcomeric proteins that increase the contractile stress and strength of the tissue, which in turn pumps more blood at higher stress throughout the developing vasculature. Feedback of beating cardiomyocytes on the expression of matrix by fibroblasts seems a reasonable model, with both synthesis and turnover of matrix and contractile elements achieving a suitable balance. Based on emerging evidence for coiled-coil biopolymers that are tension-stabilized against degradation, a minimal network model of a dynamic cell-matrix interaction is proposed. This same concept is extended to nuclear mechanics as regulated by stress on the nuclear structural proteins called lamins, which are examined in part because of the prominence of mutations in these coiled-coil proteins in diseases of the heart, amongst other organs/tissues. Variations in lamin levels during development and across adult tissues are to some extent known and appear to correlate with extracellular matrix mechanics, which we illustrate across heart, lung, and blood development. The formal perspective here on the mechanochemistry of tissue development and homeostasis could provide a useful framework for 'big data' quantitative biology, particularly of stress-sensitive differentiation, maturation, and disease processes.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
References
-
- McCain M, Parker K. Mechanotransduction: the role of mechanical stress, myocyte shape, and cytoskeletal architectre on cardiac form and function. Pflugers Arch - Eur J Physiol. 2011;462:89–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

