A new method for generating insulin-secreting cells from human pancreatic epithelial cells after islet isolation transformed by NeuroD1
- PMID: 24845703
- PMCID: PMC4060820
- DOI: 10.1089/hgtb.2013.122
A new method for generating insulin-secreting cells from human pancreatic epithelial cells after islet isolation transformed by NeuroD1
Abstract
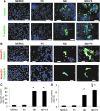
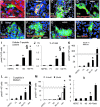
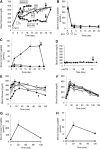
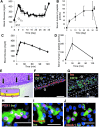
The generation of insulin-secreting cells from nonendocrine pancreatic epithelial cells (NEPEC) has been demonstrated for potential clinical use in the treatment of diabetes. However, previous methods either had limited efficacy or required viral vectors, which hinder clinical application. In this study, we aimed to establish an efficient method of insulin-secreting cell generation from NEPEC without viral vectors. We used nonislet fractions from both research-grade human pancreata from brain-dead donors and clinical pancreata after total pancreatectomy with autologous islet transplantation to treat chronic pancreatitis. It is of note that a few islets could be mingled in the nonislet fractions, but their influence could be limited. The NeuroD1 gene was induced into NEPEC using an effective triple lipofection method without viral vectors to generate insulin-secreting cells. The differentiation was promoted by adding a growth factor cocktail into the culture medium. Using the research-grade human pancreata, the effective method showed high efficacy in the differentiation of NEPEC into insulin-positive cells that secreted insulin in response to a glucose challenge and improved diabetes after being transplanted into diabetic athymic mice. Using the clinical pancreata, similar efficacy was obtained, even though those pancreata suffered chronic pancreatitis. In conclusion, our effective differentiation protocol with triple lipofection method enabled us to achieve very efficient insulin-secreting cell generation from human NEPEC without viral vectors. This method offers the potential for supplemental insulin-secreting cell transplantation for both allogeneic and autologous islet transplantation.
Figures


Similar articles
-
Neurogenic differentiation 1 directs differentiation of cytokeratin 19-positive human pancreatic nonendocrine cells into insulin-producing cells.Transplant Proc. 2010 Jul-Aug;42(6):2071-4. doi: 10.1016/j.transproceed.2010.05.114. Transplant Proc. 2010. PMID: 20692411
-
NEUROD1 Is Required for the Early α and β Endocrine Differentiation in the Pancreas.Int J Mol Sci. 2021 Jun 23;22(13):6713. doi: 10.3390/ijms22136713. Int J Mol Sci. 2021. PMID: 34201511 Free PMC article.
-
PDGF Facilitates Direct Lineage Reprogramming of Hepatocytes to Functional β-Like Cells Induced by Pdx1 and Ngn3.Cell Transplant. 2016 Oct;25(10):1893-1909. doi: 10.3727/096368916X691439. Cell Transplant. 2016. PMID: 27076404
-
Concise review: pancreas regeneration: recent advances and perspectives.Stem Cells Transl Med. 2012 Feb;1(2):150-9. doi: 10.5966/sctm.2011-0025. Epub 2012 Jan 26. Stem Cells Transl Med. 2012. PMID: 23197762 Free PMC article. Review.
-
In vitro generation of pancreatic β-cells for diabetes treatment. I. β-like cells derived from human pluripotent stem cells.Folia Histochem Cytobiol. 2019;57(1):1-14. doi: 10.5603/FHC.a2019.0001. Epub 2019 Mar 14. Folia Histochem Cytobiol. 2019. PMID: 30869153 Review.
References
-
- Baeyens L., Bonné S., Bos T., et al. (2009). Notch signaling as gatekeeper of rat acinar-to-beta-cell conversion in vitro. Gastroenterology 136, 1750–1760 - PubMed
-
- Bogdani M., Lefebvre V., Buelens N., et al. (2003). Formation of insulin-positive cells in implants of human pancreatic duct cell preparations from young donors. Diabetologia 46, 830–838 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

