Delta-Beta Coupled Oscillations Underlie Temporal Prediction Accuracy
- PMID: 24846147
- PMCID: PMC4537446
- DOI: 10.1093/cercor/bhu103
Delta-Beta Coupled Oscillations Underlie Temporal Prediction Accuracy
Abstract
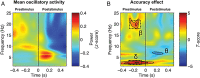
The ability to generate temporal predictions is fundamental for adaptive behavior. Precise timing at the time-scale of seconds is critical, for instance to predict trajectories or to select relevant information. What mechanisms form the basis for such accurate timing? Recent evidence suggests that (1) temporal predictions adjust sensory selection by controlling neural oscillations in time and (2) the motor system plays an active role in inferring "when" events will happen. We hypothesized that oscillations in the delta and beta bands are instrumental in predicting the occurrence of auditory targets. Participants listened to brief rhythmic tone sequences and detected target delays while undergoing magnetoencephalography recording. Prior to target occurrence, we found that coupled delta (1-3 Hz) and beta (18-22 Hz) oscillations temporally align with upcoming targets and bias decisions towards correct responses, suggesting that delta-beta coupled oscillations underpin prediction accuracy. Subsequent to target occurrence, subjects update their decisions using the magnitude of the alpha-band (10-14 Hz) response as internal evidence of target timing. These data support a model in which the orchestration of oscillatory dynamics between sensory and motor systems is exploited to accurately select sensory information in time.
Keywords: auditory; motor; neuronal oscillations; sensorimotor; timing.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Adachi Y, Shimogawara M, Higuchi M, Haruta Y, Ochiai M. 2001. Reduction of nonperiodic environmental magnetic noise in MEG measurement by continuously adjusted least square method. IEEE Trans Appl Superconduct. 11:669–672.
-
- Arnal LH, Giraud AL. 2012. Cortical oscillations and sensory predictions. Trends Cogn Sci. 16:390–398. - PubMed
-
- Arnal LH, Wyart V, Giraud AL. 2011. Transitions in neural oscillations reflect prediction errors generated in audiovisual speech. Nat Neurosci. 14:797–801. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

