Rationale and study design of the NEuroCardiac TherApy foR Heart Failure Study: NECTAR-HF
- PMID: 24846173
- PMCID: PMC4288987
- DOI: 10.1002/ejhf.80
Rationale and study design of the NEuroCardiac TherApy foR Heart Failure Study: NECTAR-HF
Abstract
Aims: Increased sympathetic activation and reduced parasympathetic tone are important pathophysiological contributors to the progression of heart failure, and are associated with poor outcome in patients. The aim of this study is to determine if vagal nerve stimulation (VNS) is a promising approach to modulate autonomic function and slow cardiac remodelling and the progression of heart failure.
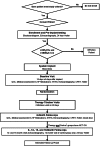
Methods: The NECTAR-HF (NEural Cardiac TherApy foR Heart Failure) trial is designed to evaluate whether the Boston Scientific VNS device is safe and may attenuate cardiac remodelling, improve cardiac function and increase exercise capacity, in symptomatic heart failure patients (New York Heart Association Class II-III) with left ventricular systolic dysfunction (ejection fraction ≤35%) and receiving optimal medical therapy. Patients will be randomized in a 2:1 ratio to receive standard optimal medical treatment plus VNS system in an active mode vs. optimal medical treatment plus VNS system in an inactive mode, for a 6 month period. After the 6 month control period, inactive VNS systems will be activated and all patients will receive VNS. The study is powered to detect differences in the primary efficacy endpoint of change in left ventricular end systolic diameter. Secondary endpoints include ejection fraction, left ventricular volumes, quality of life scores, functional capacity, and changes in biomarkers.
Conclusion: This Phase II, randomized clinical trial conducted with vagal stimulation for heart failure will provide important new information on the potential of this novel and promising technique.
Keywords: Autonomic; Cardiac; Device; Heart; Parasympathetic; Vagal.
© 2014 The Authors. European Journal of Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Figures


References
-
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics – 2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. - PMC - PubMed
-
- Yancy CW. Comprehensive treatment of heart failure: state-of-the-art medical therapy. Rev Cardiovasc Med. 2005;6(Suppl 2):S43–S57. - PubMed
-
- Mortara A, La Rovere MT, Pinna GD, Prpa A, Maestri R, Febo O, Pozzoli M, Opasich C, Tavazzi L. Arterial baroreflex modulation of heart rate in chronic heart failure. Clinical and hemodynamic correlates and prognostic implications. Circulation. 1997;96:3450–3458. - PubMed
-
- Ponikowski P, Anker SD, Chua TP, Szelemej R, Piepoli M, Adamopoulos S, Webb-Peploe K, Harrington D, Banasiak W, Wrabec K, Coats AJ. Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1997;79:1645–1650. - PubMed
-
- Schwartz PJ, De Ferrari GM, Sanzo A, Landolina M, Rordorf R, Raineri C, Campana C, Revera M, Ajmone-Marsan N, Tavazzi L, Odero A. Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Fail. 2008;10:884–891. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

