A mechanochemical switch to control radical intermediates
- PMID: 24846280
- PMCID: PMC4067147
- DOI: 10.1021/bi500050k
A mechanochemical switch to control radical intermediates
Abstract
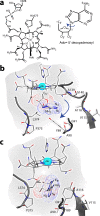
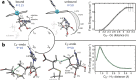
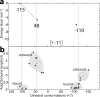
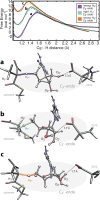
B₁₂-dependent enzymes employ radical species with exceptional prowess to catalyze some of the most chemically challenging, thermodynamically unfavorable reactions. However, dealing with highly reactive intermediates is an extremely demanding task, requiring sophisticated control strategies to prevent unwanted side reactions. Using hybrid quantum mechanical/molecular mechanical simulations, we follow the full catalytic cycle of an AdoB₁₂-dependent enzyme and present the details of a mechanism that utilizes a highly effective mechanochemical switch. When the switch is "off", the 5'-deoxyadenosyl radical moiety is stabilized by releasing the internal strain of an enzyme-imposed conformation. Turning the switch "on," the enzyme environment becomes the driving force to impose a distinct conformation of the 5'-deoxyadenosyl radical to avoid deleterious radical transfer. This mechanochemical switch illustrates the elaborate way in which enzymes attain selectivity of extremely chemically challenging reactions.
Figures
References
-
- Rètey J. (1990) Enzymic Reaction Selectivity by Negative Catalysis or How Do Enzymes Deal with Highly Reactive Intermediates?. Angew. Chem. lnt. Ed. 29, 355–361.
-
- Banerjee R.; Ragsdale S. W. (2003) The Many Faces of Vitamin B12: Catalysis by Cobalamin-Dependent Enzymes. Annu. Rev. Biochem. 72, 209–247. - PubMed
-
- Hay B. P.; Finke R. G. (1986) Thermolysis of the cobalt-carbon bond of adenosylcobalamin. 2. Products, kinetics, and cobalt-carbon bond dissociation energy in aqueous solution. J. Am. Chem. Soc. 108, 4820–4829.
-
- Hay B. P.; Finke R. G. (1987) Thermolysis of the Co-C bond in adenosylcorrins. 3. Quantification of the axial base effect in adenosylcobalamin by the synthesis and thermolysis of axial base-free adenosylcobinamide. Insights into the energetics of enzyme-assisted cobalt-carbon bond homolysis. J. Am. Chem. Soc. 109, 8012–8018.
-
- Vlasie M.; Banerjee R. (1998) Tyrosine 89 Accelerates Co-Carbon Bond Homolysis in Methylmalonyl-CoA Mutase. J. Am. Chem. Soc. 125, 5431–5435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

