Chp8, a diguanylate cyclase from Pseudomonas syringae pv. Tomato DC3000, suppresses the pathogen-associated molecular pattern flagellin, increases extracellular polysaccharides, and promotes plant immune evasion
- PMID: 24846383
- PMCID: PMC4030453
- DOI: 10.1128/mBio.01168-14
Chp8, a diguanylate cyclase from Pseudomonas syringae pv. Tomato DC3000, suppresses the pathogen-associated molecular pattern flagellin, increases extracellular polysaccharides, and promotes plant immune evasion
Abstract
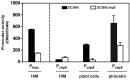
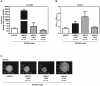
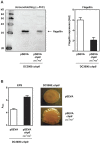
The bacterial plant pathogen Pseudomonas syringae causes disease in a wide range of plants. The associated decrease in crop yields results in economic losses and threatens global food security. Competition exists between the plant immune system and the pathogen, the basic principles of which can be applied to animal infection pathways. P. syringae uses a type III secretion system (T3SS) to deliver virulence factors into the plant that promote survival of the bacterium. The P. syringae T3SS is a product of the hypersensitive response and pathogenicity (hrp) and hypersensitive response and conserved (hrc) gene cluster, which is strictly controlled by the codependent enhancer-binding proteins HrpR and HrpS. Through a combination of bacterial gene regulation and phenotypic studies, plant infection assays, and plant hormone quantifications, we now report that Chp8 (i) is embedded in the Hrp regulon and expressed in response to plant signals and HrpRS, (ii) is a functional diguanylate cyclase, (iii) decreases the expression of the major pathogen-associated molecular pattern (PAMP) flagellin and increases extracellular polysaccharides (EPS), and (iv) impacts the salicylic acid/jasmonic acid hormonal immune response and disease progression. We propose that Chp8 expression dampens PAMP-triggered immunity during early plant infection.
Importance: The global demand for food is projected to rise by 50% by 2030 and, as such, represents one of the major challenges of the 21st century, requiring improved crop management. Diseases caused by plant pathogens decrease crop yields, result in significant economic losses, and threaten global food security. Gaining mechanistic insights into the events at the plant-pathogen interface and employing this knowledge to make crops more resilient is one important strategy for improving crop management. Plant-pathogen interactions are characterized by the sophisticated interplay between plant immunity elicited upon pathogen recognition and immune evasion by the pathogen. Here, we identify Chp8 as a contributor to the major effort of the plant pathogen Pseudomonas syringae pv. tomato DC3000 to evade immune responses of the plant.
Copyright © 2014 Engl et al.
Figures

References
-
- Food and Agriculture Organization of the United Nations 2006. World agriculture: towards 2030/2050. Interim report. Prospects for food, nutrition, agriculture and major commodity groups. Global Perspectives Studies Unit, Food and Agriculture Organization of the United Nations, Rome, Italy: http://www.fao.org/docrep/009/a0607e/a0607e00.HTM
-
- Hirano SS, Upper CD. 1990. Population biology and epidemiology of Pseudomonas syringae. Annu. Rev. Phytopathol. 28:155–177. 10.1146/annurev.phyto.28.1.155 - DOI
-
- Armstrong EL, Lindbec KD, Richardson HJ, Coombes N, O’Connor G, Matthews PW, Gaynor L. 2010. Measuring the impact of bacterial blight (Pseudomonas syringae pv. syringae) on production of Australian field pea varieties. In Dove H, Culvenor RA. (ed), Proceedings of 15th Agronomy Conference 2010, 15 to 18 November 2010 Lincoln, New Zealand http://www.regional.org.au/au/asa/2010/crop-production/legumes/7048_arms...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
