SAA drives proinflammatory heterotypic macrophage differentiation in the lung via CSF-1R-dependent signaling
- PMID: 24846388
- PMCID: PMC5395724
- DOI: 10.1096/fj.14-250332
SAA drives proinflammatory heterotypic macrophage differentiation in the lung via CSF-1R-dependent signaling
Abstract
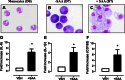
Serum amyloid A (SAA) is expressed locally in chronic inflammatory conditions such as chronic obstructive pulmonary disease (COPD), where macrophages that do not accord with the classic M1/M2 paradigm also accumulate. In this study, the role of SAA in regulating macrophage differentiation was investigated in vitro using human blood monocytes from healthy subjects and patients with COPD and in vivo using an airway SAA challenge model in BALB/c mice. Differentiation of human monocytes with SAA stimulated the proinflammatory monokines IL-6 and IL-1β concurrently with the M2 markers CD163 and IL-10. Furthermore, SAA-differentiated macrophages stimulated with lipopolysaccharide (LPS) expressed markedly higher levels of IL-6 and IL-1β. The ALX/FPR2 antagonist WRW4 reduced IL-6 and IL-1β expression but did not significantly inhibit phagocytic and efferocytic activity. In vivo, SAA administration induced the development of a CD11c(high)CD11b(high) macrophage population that generated higher levels of IL-6, IL-1β, and G-CSF following ex vivo LPS challenge. Blocking CSF-1R signaling effectively reduced the number of CD11c(high)CD11b(high) macrophages by 71% and also markedly inhibited neutrophilic inflammation by 80%. In conclusion, our findings suggest that SAA can promote a distinct CD11c(high)CD11b(high) macrophage phenotype, and targeting this population may provide a novel approach to treating chronic inflammatory conditions associated with persistent SAA expression.
Keywords: ALX/FPR2 signaling; COPD; lung inflammation; macrophage biology.
© FASEB.
Figures

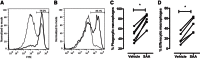
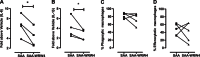
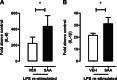



References
-
- Hashimoto D., Chow A., Noizat C., Teo P., Beasley M. B., Leboeuf M., Becker C. D., See P., Price J., Lucas D., Greter M., Mortha A., Boyer S. W., Forsberg E. C., Tanaka M., van Rooijen N., Garcia-Sastre A., Stanley E. R., Ginhoux F., Frenette P. S., Merad M. (2013) Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804 - PMC - PubMed
-
- Maus U. A., Janzen S., Wall G., Srivastava M., Blackwell T. S., Christman J. W., Seeger W., Welte T., Lohmeyer J. (2006) Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. Am. J. Respir. Cell Mol. Biol. 35, 227–235 - PubMed
-
- Shibata Y., Berclaz P. Y., Chroneos Z. C., Yoshida M., Whitsett J. A., Trapnell B. C. (2001) GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU. 1. Immunity 15, 557–567 - PubMed
-
- Dhaliwal K., Scholefield E., Ferenbach D., Gibbons M., Duffin R., Dorward D. A., Morris A. C., Humphries D., MacKinnon A., Wilkinson T. S., Wallace W. A., van Rooijen N., Mack M., Rossi A. G., Davidson D. J., Hirani N., Hughes J., Haslett C., Simpson A. J. (2012) Monocytes control second-phase neutrophil emigration in established lipopolysaccharide-induced murine lung injury. Am. J. Respir. Crit. Care Med. 186, 514–524 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

