Buprenorphine signalling is compromised at the N40D polymorphism of the human μ opioid receptor in vitro
- PMID: 24846673
- PMCID: PMC4241093
- DOI: 10.1111/bph.12785
Buprenorphine signalling is compromised at the N40D polymorphism of the human μ opioid receptor in vitro
Abstract
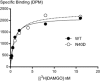
Background and purpose: There is significant variation in individual response to opioid drugs, which may result in inappropriate opioid therapy. Polymorphisms of the μ opioid receptor (MOP receptor) may contribute to individual variation in opioid response by affecting receptor function, and the effect may be ligand-specific. We sought to determine functional differences in MOP receptor signalling at several signalling pathways using a range of structurally distinct opioid ligands in cells expressing wild-type MOP receptors (MOPr-WT) and the commonly occurring MOP receptor variant, N40D.
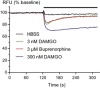
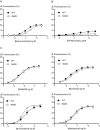
Experimental approach: MOPr-WT and MOPr-N40D were stably expressed in CHO cells and in AtT-20 cells. Assays of AC inhibition and ERK1/2 phosphorylation were performed on CHO cells, and assays of K activation were performed on AtT-20 cells. Signalling profiles for each ligand were compared between variants.
Key results: Buprenorphine efficacy was reduced by over 50% at MOPr-N40D for AC inhibition and ERK1/2 phosphorylation. Buprenorphine potency was reduced threefold at MOPr-N40D for K channel activation. Pentazocine efficacy was reduced by 50% for G-protein-gated inwardly rectifying K channel activation at MOPr-N40D. No other differences were observed for any other ligands tested.
Conclusions and implications: The N40D variant is present in 10-50% of the population. Buprenorphine is a commonly prescribed opioid analgesic, and many individuals do not respond to buprenorphine therapy. This study demonstrates that buprenorphine signalling to several effectors via the N40D variant of MOP receptors is impaired, and this may have important consequences in a clinical setting for individuals carrying the N40D allele.
© 2014 The British Pharmacological Society.
Figures




References
-
- Abrol R, Kim SK, Bray JK, Trzaskowski B, Goddard WA., 3rd Conformational ensemble view of G protein-coupled receptors and the effect of mutations and ligand binding. Methods Enzymol. 2013;520:31–48. - PubMed
-
- Befort K, Filliol D, Decaillot FM, Gaveriaux-Ruff C, Hoehe MR, Kieffer BL. A single nucleotide polymorphic mutation in the human mu-opioid receptor severely impairs receptor signaling. J Biol Chem. 2001;276:3130–3137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

