Utilization trends of anti-TNF agents and health outcomes in adults and children with inflammatory bowel diseases: a single-center experience
- PMID: 24846718
- PMCID: PMC4227810
- DOI: 10.1097/MIB.0000000000000061
Utilization trends of anti-TNF agents and health outcomes in adults and children with inflammatory bowel diseases: a single-center experience
Abstract
Background: Utilization trends and health effects of infliximab and adalimumab in inflammatory bowel disease (IBD) are incompletely understood. We aimed to describe utilization trends of these 2 anti-tumor necrosis factor (TNF) agents, determine the correlation between utilization with rates of hospitalization and surgery and describe differences in use between adults and children.
Methods: Longitudinal data were analyzed for drug utilization, hospitalization, and abdominal surgery. Descriptive statistics were used to show trends, and utilization quotients were compared for standardization. Multivariate logistic regression analysis assessed the association between drug use and rates of hospitalization and surgery.
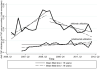
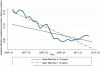
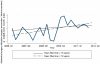
Results: Four hundred thirty-eight pediatric and 2514 adult patients with IBD generated a total of 51,882 inpatient and outpatient encounters, representing 1185 Crohn's disease, 1531 ulcerative colitis, and 236 indeterminate colitis patients. From 2007 through 2012, utilization quotients declined for hospitalization but remained unchanged for surgery; adalimumab saw a 3-fold increase, despite continued dominance of infliximab. Median band and mean fitted plots showed downward hospitalization trends from 2006 to 2012. Utilization of infliximab peaked in 2008, Q4 with gradual decline to 2012, Q2; and adalimumab showed moderate increased utilization since 2007, Q1. Use of infliximab (odds ratio [OR], 0.76; 95% confidence interval [CI], 0.70-0.83) and adalimumab (OR, 0.79; 95% CI, 0.72-0.87) was associated with decreased hospitalization risk but not associated with reduced abdominal surgery risk. Children had increased hospitalization (OR, 2.68; 95% CI, 2.49-2.88) but decreased risk for abdominal surgery (OR, 0.57; 95% CI, 0.46-0.70).
Conclusions: Current infliximab use remains substantially greater than adalimumab use, despite recent increased use of adalimumab. Although trends for hospitalization for IBD are decreasing, it is not reflected in abdominal surgery rates in a tertiary IBD referral center.
Figures
References
-
- Hanauer SB, Feagan BG, Lichtenstein GR, et al. ACCENT I Study Group. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. - PubMed
-
- Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885. - PubMed
-
- Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. - PubMed
-
- Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333. quiz 591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources