The bacterial phosphoenolpyruvate:carbohydrate phosphotransferase system: regulation by protein phosphorylation and phosphorylation-dependent protein-protein interactions
- PMID: 24847021
- PMCID: PMC4054256
- DOI: 10.1128/MMBR.00001-14
The bacterial phosphoenolpyruvate:carbohydrate phosphotransferase system: regulation by protein phosphorylation and phosphorylation-dependent protein-protein interactions
Abstract
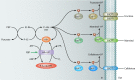
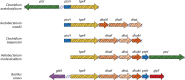
The bacterial phosphoenolpyruvate (PEP):carbohydrate phosphotransferase system (PTS) carries out both catalytic and regulatory functions. It catalyzes the transport and phosphorylation of a variety of sugars and sugar derivatives but also carries out numerous regulatory functions related to carbon, nitrogen, and phosphate metabolism, to chemotaxis, to potassium transport, and to the virulence of certain pathogens. For these different regulatory processes, the signal is provided by the phosphorylation state of the PTS components, which varies according to the availability of PTS substrates and the metabolic state of the cell. PEP acts as phosphoryl donor for enzyme I (EI), which, together with HPr and one of several EIIA and EIIB pairs, forms a phosphorylation cascade which allows phosphorylation of the cognate carbohydrate bound to the membrane-spanning EIIC. HPr of firmicutes and numerous proteobacteria is also phosphorylated in an ATP-dependent reaction catalyzed by the bifunctional HPr kinase/phosphorylase. PTS-mediated regulatory mechanisms are based either on direct phosphorylation of the target protein or on phosphorylation-dependent interactions. For regulation by PTS-mediated phosphorylation, the target proteins either acquired a PTS domain by fusing it to their N or C termini or integrated a specific, conserved PTS regulation domain (PRD) or, alternatively, developed their own specific sites for PTS-mediated phosphorylation. Protein-protein interactions can occur with either phosphorylated or unphosphorylated PTS components and can either stimulate or inhibit the function of the target proteins. This large variety of signal transduction mechanisms allows the PTS to regulate numerous proteins and to form a vast regulatory network responding to the phosphorylation state of various PTS components.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Erni B. 2013. The bacterial phosphoenolpyruvate:sugar phosphotransferase system (PTS): an interface between energy and signal transduction. J. Iran. Chem. Soc. 10:593–630. 10.1007/s13738-012-0185-1 - DOI
-
- Pickl A, Johnsen U, Schönheit P. 2012. Fructose degradation in the haloarchaeon Haloferax volcanii involves a bacterial type phosphoenolpyruvate-dependent phosphotransferase system, fructose-1-phosphate kinase, and class II fructose-1,6-bisphosphate aldolase. J. Bacteriol. 194:3088–3097. 10.1128/JB.00200-12 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

