N-linked glycosylation in Archaea: a structural, functional, and genetic analysis
- PMID: 24847024
- PMCID: PMC4054257
- DOI: 10.1128/MMBR.00052-13
N-linked glycosylation in Archaea: a structural, functional, and genetic analysis
Abstract
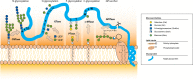
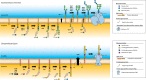
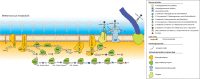
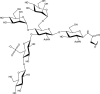
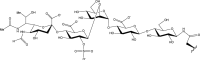
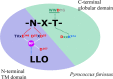
N-glycosylation of proteins is one of the most prevalent posttranslational modifications in nature. Accordingly, a pathway with shared commonalities is found in all three domains of life. While excellent model systems have been developed for studying N-glycosylation in both Eukarya and Bacteria, an understanding of this process in Archaea was hampered until recently by a lack of effective molecular tools. However, within the last decade, impressive advances in the study of the archaeal version of this important pathway have been made for halophiles, methanogens, and thermoacidophiles, combining glycan structural information obtained by mass spectrometry with bioinformatic, genetic, biochemical, and enzymatic data. These studies reveal both features shared with the eukaryal and bacterial domains and novel archaeon-specific aspects. Unique features of N-glycosylation in Archaea include the presence of unusual dolichol lipid carriers, the use of a variety of linking sugars that connect the glycan to proteins, the presence of novel sugars as glycan constituents, the presence of two very different N-linked glycans attached to the same protein, and the ability to vary the N-glycan composition under different growth conditions. These advances are the focus of this review, with an emphasis on N-glycosylation pathways in Haloferax, Methanococcus, and Sulfolobus.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures







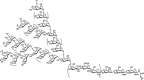







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

