Aurora A orchestrates entosis by regulating a dynamic MCAK-TIP150 interaction
- PMID: 24847103
- PMCID: PMC4034728
- DOI: 10.1093/jmcb/mju016
Aurora A orchestrates entosis by regulating a dynamic MCAK-TIP150 interaction
Abstract
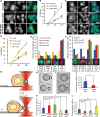
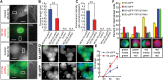
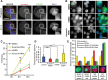
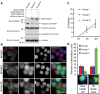
Entosis, a cell-in-cell process, has been implicated in the formation of aneuploidy associated with an aberrant cell division control. Microtubule plus-end-tracking protein TIP150 facilitates the loading of MCAK onto the microtubule plus ends and orchestrates microtubule plus-end dynamics during cell division. Here we show that TIP150 cooperates with MCAK to govern entosis via a regulatory circuitry that involves Aurora A-mediated phosphorylation of MCAK. Our biochemical analyses show that MCAK forms an intra-molecular association, which is essential for TIP150 binding. Interestingly, Aurora A-mediated phosphorylation of MCAK modulates its intra-molecular association, which perturbs the MCAK-TIP150 interaction in vitro and inhibits entosis in vivo. To probe if MCAK-TIP150 interaction regulates microtubule plasticity to affect the mechanical properties of cells during entosis, we used an optical trap to measure the mechanical rigidity of live MCF7 cells. We find that the MCAK cooperates with TIP150 to promote microtubule dynamics and modulate the mechanical rigidity of the cells during entosis. Our results show that a dynamic interaction of MCAK-TIP150 orchestrated by Aurora A-mediated phosphorylation governs entosis via regulating microtubule plus-end dynamics and cell rigidity. These data reveal a previously unknown mechanism of Aurora A regulation in the control of microtubule plasticity during cell-in-cell processes.
Keywords: Aurora A; MCAK; TIP150; entosis; kinesin; microtubule plus-end.
© The Author (2014). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, IBCB, SIBS, CAS. All rights reserved.
Figures


Similar articles
-
TIP150 interacts with and targets MCAK at the microtubule plus ends.EMBO Rep. 2009 Aug;10(8):857-65. doi: 10.1038/embor.2009.94. Epub 2009 Jun 19. EMBO Rep. 2009. PMID: 19543227 Free PMC article.
-
The Microtubule Plus End Tracking Protein TIP150 Interacts with Cortactin to Steer Directional Cell Migration.J Biol Chem. 2016 Sep 23;291(39):20692-706. doi: 10.1074/jbc.M116.732719. Epub 2016 Jul 22. J Biol Chem. 2016. PMID: 27451391 Free PMC article.
-
Regulation of a dynamic interaction between two microtubule-binding proteins, EB1 and TIP150, by the mitotic p300/CBP-associated factor (PCAF) orchestrates kinetochore microtubule plasticity and chromosome stability during mitosis.J Biol Chem. 2013 May 31;288(22):15771-85. doi: 10.1074/jbc.M112.448886. Epub 2013 Apr 17. J Biol Chem. 2013. PMID: 23595990 Free PMC article.
-
Mitotic centromere-associated kinesin (MCAK): a potential cancer drug target.Oncotarget. 2011 Dec;2(12):935-47. doi: 10.18632/oncotarget.416. Oncotarget. 2011. PMID: 22249213 Free PMC article. Review.
-
Mitosis: MCAK under the aura of Aurora B.Curr Biol. 2004 May 4;14(9):R346-8. doi: 10.1016/j.cub.2004.04.022. Curr Biol. 2004. PMID: 15120087 Review.
Cited by
-
Mitotic motor CENP-E cooperates with PRC1 in temporal control of central spindle assembly.J Mol Cell Biol. 2020 Aug 1;12(8):654-665. doi: 10.1093/jmcb/mjz051. J Mol Cell Biol. 2020. PMID: 31174204 Free PMC article.
-
Bridging cells of three colors with two bio-orthogonal click reactions.Chem Sci. 2015 Nov 1;6(11):6425-6431. doi: 10.1039/c5sc01315a. Epub 2015 Jul 28. Chem Sci. 2015. PMID: 28757958 Free PMC article.
-
KIF2C/MCAK a prognostic biomarker and its oncogenic potential in malignant progression, and prognosis of cancer patients: a systematic review and meta-analysis as biomarker.Crit Rev Clin Lab Sci. 2024 Sep;61(6):404-434. doi: 10.1080/10408363.2024.2309933. Epub 2024 Feb 12. Crit Rev Clin Lab Sci. 2024. PMID: 38344808 Free PMC article.
-
Entosis is induced by ultraviolet radiation.iScience. 2021 Jul 24;24(8):102902. doi: 10.1016/j.isci.2021.102902. eCollection 2021 Aug 20. iScience. 2021. PMID: 34401679 Free PMC article.
-
Liquid-liquid phase separation of microtubule-binding proteins in the regulation of spindle assembly.Cell Prolif. 2024 Oct;57(10):e13649. doi: 10.1111/cpr.13649. Epub 2024 May 13. Cell Prolif. 2024. PMID: 38736355 Free PMC article. Review.
References
-
- Abodief W.T., Dey P., Al-Hattab O. Cell cannibalism in ductal carcinoma of breast. Cytopathology. 2006;17:304–305. - PubMed
-
- Akhmanova A., Steinmetz M.O. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 2008;9:309–322. - PubMed
-
- Andrews P.D., Ovechkina Y., Morrice N., et al. Aurora B regulates MCAK at the mitotic centromere. Dev. Cell. 2004;6:253–268. - PubMed
-
- Buosciolo A., Pesce G., Sasso A. New calibration method for position detector for simultaneous measurements of force constants and local viscosity in optical tweezers. Opt. Commun. 2004;230:357–368.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

