A randomized, prospective, intercontinental evaluation of a bioresorbable polymer sirolimus-eluting coronary stent system: the CENTURY II (Clinical Evaluation of New Terumo Drug-Eluting Coronary Stent System in the Treatment of Patients with Coronary Artery Disease) trial
- PMID: 24847155
- PMCID: PMC4200026
- DOI: 10.1093/eurheartj/ehu210
A randomized, prospective, intercontinental evaluation of a bioresorbable polymer sirolimus-eluting coronary stent system: the CENTURY II (Clinical Evaluation of New Terumo Drug-Eluting Coronary Stent System in the Treatment of Patients with Coronary Artery Disease) trial
Abstract
Aim: The aim of this study was to establish safety and efficacy of a new sirolimus-eluting stent with bioresorbable polymer, Ultimaster (BP-SES). Sirolimus-eluting stent with bioresorbable polymer was compared with everolimus-eluting, permanent polymer, Xience stent (PP-EES) in the frame of a CENTURY II clinical trial designed to make global clinical data compliant with regulatory requirements in Europe and Japan.
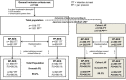
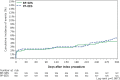
Methods and results: The CENTURY II is a prospective, multicentre, randomized (1 : 1), single blind, controlled, non-inferiority clinical trial conducted at 58 study sites in Japan, Europe, and Korea. A total of 1123 patients requiring a percutaneous coronary intervention (PCI) procedure, with implantation of drug-eluting stent (DES), were enrolled [total population (TP)]. Randomization of patients was stratified for the subset of patients matching requirements for DES in Japan (Cohort JR, n = 722). Baseline patient demographic and angiographic characteristics were similar in both study arms, with minimal differences between the TP and Cohort JR. The primary endpoint, freedom from target lesion failure (TLF) at 9 months-TLF [composite of cardiac death, target-vessel-related myocardial infarction (MI) and target lesion revascularization]-was 95.6% with BP-SES and 95.1% with PP-EES (Pnon-inferiority<0.0001). Composite of cardiac death and MI rate was 2.9 and 3.8% (P = 0.40) and target vessel revascularization was 4.5% with BP-SES and 4.2% with PP-EES (P = 0.77). The stent thrombosis rate was 0.9% in both arms. In Cohort JR, freedom from TLF was 95.9 and 94.6% (Pnon-inferiority < 0.0005) with BP-SES and PP-EES, respectively.
Conclusion: The new bioresorbable polymer sirolimus-eluting stent showed safety and efficacy profiles similar to durable polymer everolimus-eluting stent at 9-month follow-up.
Study registration number: UMIN000006940.
Keywords: Bioresorbable polymer; Drug-eluting stent; Everolimus; Randomized trial; Sirolimus.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2014. For permissions please email: journals.permissions@oup.com.
Figures

Comment in
-
Drug-eluting stent technology: progress beyond the polymer.Eur Heart J. 2014 Aug 7;35(30):1991-5. doi: 10.1093/eurheartj/ehu224. Epub 2014 Jun 7. Eur Heart J. 2014. PMID: 24908391 No abstract available.
References
-
- Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE. SIRIUS Investigators. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. - PubMed
-
- Stone GW, Midei M, Newman W, Sanz M, Hermiller JB, Williams J, Farhat N, Mahaffey KW, Cutlip DE, Fitzgerald PJ, Sood P, Su X, Lansky AJ. SPIRIT III Investigators. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomised trial. JAMA. 2008;299:1903–1913. - PubMed
-
- Jeremias A, Kirtane A. Balancing efficacy and safety of drug-eluting stents in patients undergoing percutaneous coronary intervention. Ann Intern Med. 2008;148:234–238. - PubMed
-
- Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schömig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS, Goy JJ, Park SJ, Sabaté M, Suttorp MJ, Kelbaek H, Spaulding C, Menichelli M, Vermeersch P, Dirksen MT, Cervinka P, Petronio AS, Nordmann AJ, Diem P, Meier B, Zwahlen M, Reichenbach S, Trelle S, Windecker S, Jüni P. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937–948. - PubMed
-
- Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation. 2007;115:1440–1455. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

