Regulation of Desmocollin3 Expression by Promoter Hypermethylation is Associated with Advanced Esophageal Adenocarcinomas
- PMID: 24847386
- PMCID: PMC4026999
- DOI: 10.7150/jca.9145
Regulation of Desmocollin3 Expression by Promoter Hypermethylation is Associated with Advanced Esophageal Adenocarcinomas
Abstract
Background: Desmocollin3 (DSC3) is a member of the cadherin superfamily of calcium-dependent cell adhesion molecules and plays an important role in tumor invasion and metastasis. In this study, we investigated the epigenetic mechanism that regulates DSC3 expression in esophageal adenocarcinomas (EACs).
Methods: Expression of DSC3 was analyzed by quantitative real-time polymerase chain reaction (qRT-PCR). The promoter DNA methylation level of DSC3 was examined using quantitative bisulfite pyrosequencing.
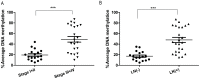
Results: The qRT-PCR analysis demonstrated significant down-regulation of the DSC3 mRNA levels in human EAC cell lines and tissue samples (P<.001). In addition, the EAC cell lines and tumor samples have aberrant promoter hypermethylation as compared to normal esophageal samples (P<.001). DSC3 promoter hypermethylation (>10% methylation level) was detected in 97.5% (39/40) of EAC samples whereas none of the normal tissue samples showed hypermethylation (P<.0001). There was a significant inverse correlation between promoter DNA methylation levels and mRNA expression folds for DSC3 (coefficient r=-0.685, P<.0001). Treatment of FLO-1 and SKGT4 EAC cells with 5-Aza-deoxytidine led to a significant reduction in the promoter DNA methylation levels with restoration of the DSC3 expression, suggesting that promoter DNA methylation is a key epigenetic mechanism regulating DSC3 expression. High DSC3 promoter DNA methylation levels were significantly correlated with advanced tumor stage (P<.001) and lymph node metastasis (P<.001).
Conclusion: Taken together, our results demonstrate that epigenetic silencing of DSC3 is a frequent finding in EAC that is possibly associated with advanced stages.
Keywords: DSC3; cancer; epigenetics; esophageal; metastasis..
Conflict of interest statement
Competing Interests: All the authors declared no conflict of interest for the purpose of this study.
Figures





References
-
- Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–12. doi:10.1016/S0140-6736(12)60643-6. - PubMed
-
- Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. Journal of the National Cancer Institute. 2005;97:142–6. doi:10.1093/jnci/dji024. - PubMed
-
- Carr JS, Zafar SF, Saba N, Khuri FR, El-Rayes BF. Risk factors for rising incidence of esophageal and gastric cardia adenocarcinoma. Journal of gastrointestinal cancer. 2013;44:143–51. doi:10.1007/s12029-013-9480-z. - PubMed
-
- Lassen A, Hallas J, de Muckadell OB. Esophagitis: incidence and risk of esophageal adenocarcinoma--a population-based cohort study. Am J Gastroenterol. 2006;101:1193–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

